Cell>Point of Centennial, CO, has submitted an investigational new drug (IND) application to the U.S. Food and Drug Administration (FDA) for a radiopharmaceutical for imaging coronary artery disease.
The IND covers a phase Ib/II trial of the company's technetium-99m-labeled glucosamine (Tc-99m EC-G) diagnostic imaging agent. It is designed to evaluate patients with cardiovascular issues, such as reduced blood supply (ischemia), recent heart attacks, and questionable heart muscle cell viability.
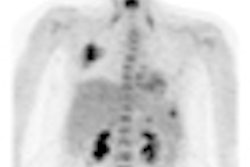
The study will attempt to determine if Tc-99m EC-G can be used as an alternative to traditional myocardial perfusion imaging (MPI) agents, and be target-specific for the region of ischemia.
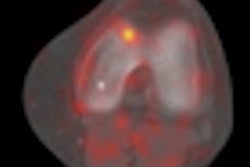
Preclinical studies have shown that the agent targets and concentrates in ischemic heart muscle tissue, according to Cell>Point.
Related Reading
Cell>Point touts SPECT agent results at SNM, June 9, 2010
Cell>Point names Rollo as president, September 13, 2006
Biotech firm Cell>Point sees promise for oncology SPECT, May 20, 2005
Philips adds molecular imaging partnership, September 29, 2003
Copyright © 2010 AuntMinnie.com