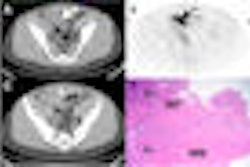
The U.S. Centers for Medicare and Medicaid Services (CMS) has begun taking feedback on a proposal to cover F-18 sodium fluoride (F-18 NaF) PET imaging studies to identify bone metastasis of cancer under certain conditions.
The agency proposal would cover F-18 NaF PET imaging when the patent's physician determines that the study could help initial antitumor treatment strategy or guide subsequent antitumor treatment strategy after the completion of initial treatment, if the patient and the F-18 NaF PET provider are enrolled in a prospective clinical study.
The F-18 NaF PET clinical study must be designed to collect additional information at the time of the scan to assist in initial treatment planning and in identifying symptomatic bone metastases.
CMS is taking public comments until December 30, with a decision scheduled for the end of February 2010.
Related Reading
F-18 fluoride PET/CT may better manage painful bone metastases, November 29, 2009
Early FDG-PET/CT may help head and neck cancer therapy, November 20, 2009
Fluoride ion PET/CT beats FDG for metastatic spinal bone lesions, November 3, 2009
FDG-PET/CT scans spot head and neck cancer return, October 14, 2009
FDG-PET/CT helps track pediatric bone and soft-tissue tumors, June 16, 2009
Copyright © 2009 AuntMinnie.com