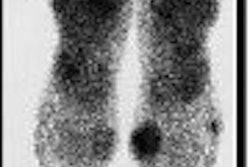
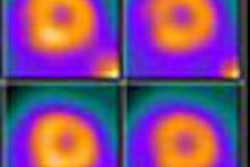
GE Healthcare is enrolling patients in a clinical trial designed to evaluate cardiac scans using the iodine-123 (I-123) MIBG radiopharmaceutical.
The study is designed to validate information provided by the scans in evaluating the extent and nature of nerve damage, and to help physicians determine individual risk for sudden cardiac death, according to the Chalfont St. Giles, U.K.-based firm.
The trial seeks to assess the nerves that regulate the rate and strength of the heart's contraction; quantitative myocardial I-123 MIBG uptake will be used to identify heart failure subjects at higher risk of earlier heart failure progression, potentially fatal ventricular arrhythmias, or cardiac death, GE said.
Study participants must be adults with an established diagnosis of heart failure (New York Heart Association Class II or III) and reduced left ventricular ejection fraction (LVEF) (< 35%), according to the firm.
By AuntMinnie.com staff writers
November 14, 2006
Related Reading
Boston Scientific, GE integrate informatics apps, November 9, 2006
Road to RSNA, GE Healthcare, November 9, 2006
Road to RSNA, GE Healthcare, November 8, 2006
Road to RSNA, GE Healthcare, November 7, 2006
Road to RSNA, GE Healthcare, November 6, 2006
Copyright © 2006 AuntMinnie.com