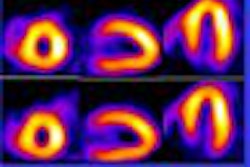
Bristol-Myers Squibb Medical Imaging and development partner Adenosine Therapeutics have initiated phase II clinical trials for BMS068645 (also known as ATL146e), a selective A2a adenosine receptor agonist designed for use as a pharmacologic stress agent in cardiac perfusion imaging studies.
Adenosine Therapeutics of Charlottesville, VA, granted a worldwide license in April 2000 to the North Billerica, MA-based firm for the development and use of the agent for the diagnosis and prognosis of coronary artery disease. Bristol-Myers Squibb is conducting and funding all clinical trials in cardiac imaging, and licensing select data back to Adenosine for use outside of cardiac imaging.
March 24, 2004
Related Reading
Kereos, Bristol-Myers Squibb to collaborate in molecular imaging, March 3, 2004
Fujisawa, Bristol-Myers Squibb ink promotion deal, October 21, 2003
DraxImage, Bristol-Meyers Squibb extend partnership, July 2, 2003
AnorMed, Bristol-Myers Squibb link up, December 10, 2002
Bristol-Myers Squibb appoints imaging chief, July 31, 2002
Copyright © 2004 AuntMinnie.com