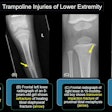
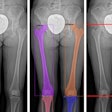
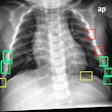
Digital radiography (DR) developer Swissray International has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its ddRCompact series of DR systems.
Suitable for orthopedics, smaller hospitals, imaging centers, as well as veterinary and chiropractic applications, ddRCompact features Swissray's Automated Positioning System (APS), AutoStitching for orthopedic examinations, remote monitoring, and a 99% uptime guarantee, according to the Elizabeth, NJ-based vendor.
By AuntMinnie.com staff writers
June 5, 2007
Related Reading
Swissray, Stryker sign OEM pact, February 8, 2007
Swissray launches ddRCompact DR system, December 4, 2006
Mikron, Swissray partner, November 27, 2006
Road to RSNA, Swissray International, October 30, 2006
Swissray gets Brigham and Women's install, October 3, 2006
Copyright © 2007 AuntMinnie.com