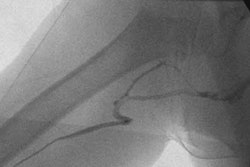
Interventional radiology, vascular surgery, and cardiology device developer IDev Technologies said the Food and Drug Administration has given marketing clearance for the Texan Longhorn foreign body retrieval device, the latest addition to IDev's Texan product line.
The Texan Longhorn is a 120-cm, 6 French system with 0.018-inch guidewire compatibility, enabling an interventionalist to use the device in distal peripheral applications while maintaining access throughout the entire procedure, according to Houston-based IDev.
The product features a variable loop design, which allows for loop size adjustment within the vessel and the capture or manipulation of a foreign body without having to determine the appropriately sized device prior to the procedure, IDev said.
The firm also said it has released a CE Mark version of the Texan Longhorn into the European market.
By AuntMinnie.com staff writers
May 18, 2005
Copyright © 2005 AuntMinnie.com