Electromagnetic localization developer Calypso Medical Technologies has completed the second stage of an investigational device exemption (IDE) clinical study for its Calypso 4D Localization System.
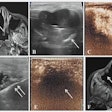
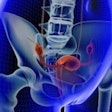
Using magnetic wireless markers implanted in or near a tumor, the Calypso system is designed to provide continuous confirmation of a target's location for radiation therapy treatment setup and during radiation delivery.
Researchers used the Calypso system to verify the location of the target in a number of prostate cancer patients implanted with the markers, the Seattle-based company reported from the American Association of Physicists in Medicine's (AAPM) annual meeting in Pittsburgh.
The company plans to conduct stage III of the IDE trial during the fourth quarter of this year and is anticipating approval of the Calypso 4D Localization System sometime in 2005, Calypso said.
By AuntMinnie.com staff writersJuly 28, 2004
Copyright © 2004 AuntMinnie.com