GE Healthcare has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its new cardiac imaging platform, the Discovery CT750 HD Freedom Edition.
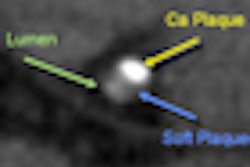
The cardiac spectral CT system features Motion Freedom with intelligent motion correction via SnapShot Freeze; Calcium Freedom with enhanced coronary visualization using Gemstone Spectral Imaging (GSI) Cardiac; and Horizon Free for plaque material composition assessment and perfusion calculations.
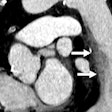
GE's SnapShot Freeze technique is designed to reduce coronary motion and to detect vessel motion and velocity. SnapShot Freeze can help determine vessel position and correct the effects of motion during cardiac CT exams.
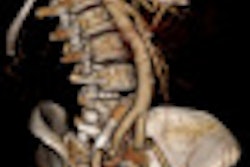
The HD Freedom Edition's first U.S. installation was at the University of Washington in Seattle. The CT scanner is installed at two dozen locations in Europe and Japan.