The Food and Drug Administration has accepted a new drug application (NDA) from Advanced Magnetics for its Combidex MRI contrast agent. Since the agent was accorded priority review status earlier this month, the NDA is scheduled to be completed by June 19, according to the Cambridge, MA-based firm. The FDA has notified Advanced Magnetics that it is contemplating taking the NDA to the agency's medical imaging advisory committee in May.
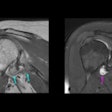
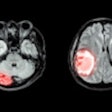
The NDA includes two indications for Combidex. The first is for the diagnosis of lymph node disease to assist in directing biopsy and surgery, and to aid in the staging of metastatic lymph node involvement for a variety of cancers, including breast and prostate cancer. The product's second indication is for the detection, diagnosis,and characterization of benign versus malignant lesions of the liver and spleen.
By AuntMinnie.com staff writersFebruary 24, 2000
Copyright © 2000 AuntMinnie.com