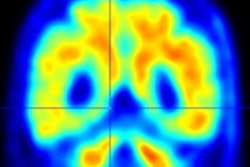
Using PET to assess amyloid beta and tau biomarkers, researchers have found in a July 30 JAMA Neurology study that even in individuals without cognitive impairment, these biomarkers are associated with near-term progression to mild cognitive impairment (MCI) and long-term cognitive decline.
The longitudinal study used data from four independent population-based cohorts and was led by Cherie Strikwerda-Brown, PhD, from the psychiatry department at McGill University in Montreal, Canada.
She and her colleagues found that in most older people without cognitive impairment but in whom PET indicated the presence of amyloid beta and tau biomarkers as well as neurodegeneration, the individuals developed Alzheimer's disease (AD) symptoms within two to three years (JAMA Neurology, July 30, 2022).
The U.S. National Institute on Aging-Alzheimer's Association (NIA-AA) revised its research criteria for Alzheimer's disease in 2018 to add tau biomarkers and said the presence of amyloid beta and tau biomarkers are also needed for the diagnosis of AD. In addition, neurodegeneration (in other words, cortical thickness) is used to stage disease severity. That means individuals who have abnormal amyloid beta and tau levels can have biological AD, even if they do not show cognitive-decline symptoms.
Researchers have debated the clinical significance of biologically defined Alzheimer's, particularly because abnormal levels of these biomarkers are present in approximately 20% of older adults without cognitive impairment both in vivo and at autopsy. However, because most of the cited studies have been cross-sectional, it has been unclear whether individuals with abnormal amyloid and tau levels are at imminent risk of developing AD-related cognitive impairment.
In the current study, Strikwerda-Brown and colleagues sought to make that determination by using PET to assess amyloid beta and tau deposition in four independent cohorts.
The researchers included a total of 580 participants from the family history-positive Pre-symptomatic Evaluation of Experimental or Novel Treatments for Alzheimer's Disease (PREVENT-AD) cohort, the Harvard Aging Brain Study (HABS), the Australian Imaging, Biomarker and Lifestyle (AIBL) study, and the Knight Alzheimer Disease Research Center (ADRC) dataset.
Every participant had at least one amyloid beta PET scan using F-18 NAV4694, C-11 Pittsburgh Compound B, F-18 AV45 (florbetapir), or a combination of both. Tau PET was performed using F-18 AV1451 on all patients and T1-weighted structural MRI scans were collected on 3-tesla scanners.
Patients were cognitively unimpaired at the time of their PET scans and had at least 12 months of clinical follow-up. Assessing global amyloid burden (A) and a composite temporal region of tau PET uptake (T), participants were sorted into four groups:
- A+T+, positive for amyloid and tau
- A+T−, positive for amyloid, negative for tau
- A−T+, negative for amyloid, positive for tau
- A−T−, negative for amyloid, negative for tau
The presence or absence of neurodegeneration (N) was also assessed.
The researchers found 33% to 83% of individuals without cognitive impairment and with abnormal elevation of the biomarkers (A+T+) progressed to MCI within two to three years after PET scanning. The numbers increased across all cohorts when restricted to individuals who were positive for neurodegeneration (A+T+N+) and reached a progression rate of 43% to 100%.
In addition, the remaining participants with abnormal biomarker levels also experienced cognitive decline, which suggests they too are on a pathway toward AD symptoms.
The study authors also found positivity of both amyloid beta and tau PET (A+T+) was associated with a seven to 29 times greater hazard of progression from cognitively unimpaired to showing signs of MCI, compared with a positive amyloid beta scan without tau positivity (A+T−).
"These findings support the clinical validity of a biological definition of AD in participants without cognitive impairment that is based on the presence of both amyloid beta and tau," they wrote. "When preventive treatments become available, the use of such a biological definition of AD to identify persons with probable preclinical AD could substantially mitigate the AD epidemic. Until then, elevations in both amyloid beta and tau PET indicate imminent clinical progression in most individuals without cognitive impairment."
Not only is the finding relevant for treating current patients, but it has implications for future trials, the researchers said. However, because the majority of participants were non-Hispanic white individuals, more studies are needed to determine if Strikwerda-Brown and colleagues' findings are applicable to other groups.