CHICAGO - A new line of MRI scanners, as well as new offerings in x-ray and ultrasound and enhancements to other modalities, are among the highlights for GE Healthcare at this year's RSNA meeting.
MRI
GE is highlighting a trio of new MRI scanners at this year's meeting, all of which are based on a new productivity platform called SignaWorks.
SignaWorks includes specific applications for areas such as neurology, body, cardiac, musculoskeletal, oncology, and pediatric imaging. It also features four application tools: HyperWorks, a speed scanning tool; SilentWorks, a noise-reducing technology; ViosWorks, a cardiac imaging technique that visualizes and quantifies 4D flow and features a work-in-progress application for deep learning in MRI; and ImageWorks, a tool that boosts MR performance through automation and advanced postprocessing capabilities.
On the hardware side, one of the new scanners is Signa Voyager, a 1.5-tesla unit shown as a work-in-progress at RSNA 2015. It includes the company's next-generation technology in radiofrequency (RF) coils and gradients, and it has one of the smallest footprints and lowest power consumption for a system of its type, making it a good first 1.5-tesla wide-bore unit for new MRI users, according to the firm.
Signa Architect, a 3-tesla system, and Signa Artist, a 1.5-tesla device, are both wide-bore scanners supported by 96-channel and 128-channel RF coil designs. Signa Architect also features a work-in-progress 48-channel head coil that supports applications such as video goggles for functional MRI exams and includes an EEG-compatible design for advanced neuroimaging.
As a work-in-progress, GE is demonstrating Signa Premier, a wide-bore 3-tesla scanner. The system sports powerful 154-channel gradients and a 70-cm bore, and it is designed to enable sites to perform both high-end research work as well as a patient-friendly environment for clinical use.
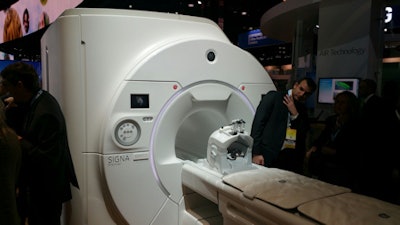 Signa Premier is a 3-tesla MRI scanner for research and clinical use being shown as a work-in-progress.
Signa Premier is a 3-tesla MRI scanner for research and clinical use being shown as a work-in-progress.The company is also highlighting its Signa Lift in-field upgrade program for 1.5-tesla and 3-tesla units that allows clinicians to upgrade their existing units to the SignaWorks platform. By upgrading, customers can save up to 50% in construction costs and up to 50% in equipment costs, compared with a new GE 1.5- or 3-tesla system, and realize an up to 30% increase in procedures due to increased throughput, according to the firm.
Finally, GE lifted the veil on Freelium, a project the company is working on to develop a fully sealed magnet that does not require helium refills for cryogen. The Freelium design is based on 20 L of cryogen that is recirculated in an enclosed loop. Freelium will be used in future GE magnet designs; the company is showing it as a work-in-progress.
CT
In CT, GE is showing new technologies based on R&D investments in its Revolution product family. A major new launch is RevolutionCT with Gemstone spectral imaging (GSI) Xtream, which enables volume scanning using the GSI spectral imaging technology. RevolutionCT GSI Xtream now supports volume spectral scanning at a width of 80 mm, compared with the 40 mm width on other systems in the GE product line.
Volume spectral scanning enables better clinical outcomes, and an evaluation site in China that is using the system is experiencing both higher patient throughput and changes in their diagnostic decisions. RevolutionCT GSI Xtream is pending 510(k) clearance from the U.S. Food and Drug Administration (FDA).
 GE is highlighting new enhancements on its Logiq E9 ultrasound scanner.
GE is highlighting new enhancements on its Logiq E9 ultrasound scanner.GE is also showing enhancements to other CT scanners in its product line, including Revolution HD, which is getting new metal artifact and iterative reconstruction algorithms, and Revolution Evo, which has received new dose-optimized CT protocols.
Ultrasound
GE has revamped its Logiq E9 XDclear 2.0 system, adding a new XDclear processing engine, a new widescreen display, and what GE calls agile acoustic architecture. The new platform has improved the performance of Logiq E9 XDC 2.0, with 22% more contrast, 99% improved spatial resolution, and 170% more image information, compared with Logiq E9 XDclear, according to the firm.
In its booth, GE is also highlighting Logiq S7 with XDclear, which now features the company's XDclear transducers; Logiq P9, which increases the speed of exams by 32% and, due to a streamlined interface, reduces keystrokes by 44%; and Voluson E10 with HDlive technology, which includes a curved matrix electronic 4D probe designed for ob/gyn applications.
GE is also showcasing ViewPoint 6, an ultrasound IT package that gives clinicians comprehensive patient and exam data in one view, and Invenia ABUS, an automated breast ultrasound system (ABUS) that has shown a 55% increase in invasive breast cancer detection in women with dense tissue, compared with mammography alone, according to the firm.
Women's imaging
GE's big news in women's imaging this year is Senographe Pristina, a mammography system designed to reduce the discomfort and pain of a mammogram, and Seno Iris, a new workstation that speeds up mammography diagnoses. Both technologies were first shown at the European Congress of Radiology (ECR) in March.
 Senographe Pristina includes a feature that allows women to control their own breast compression.
Senographe Pristina includes a feature that allows women to control their own breast compression.Rather than compress the breast automatically, Senographe Pristina features a self-compression tool that patients manage with a remote control device, as well as a rounded bucky intended to reduce a woman's discomfort by mitigating the tendency to tense the pectorals while holding conventional handgrips. Instead, patients lean on armrests, allowing the muscles to relax and simplifying positioning, according to the firm.
GE worked with patients, technologists, and radiologists from Gustave Roussy Cancer Center in Villejuif, France, to develop the system. The Pristina gantry is available for order in the U.S. and Europe, while the self-compression feature is pending FDA 510(k) clearance in the U.S. but is available in Europe. The device is designed to be upgraded to digital breast tomosynthesis (DBT) applications; in August, GE submitted a premarket approval supplement for DBT for Pristina.
GE's new mammography workstation, Seno Iris, is a vendor-neutral platform that can be used alone or integrated into a facility's PACS. It features dedicated tools such as image marking, reconstruction, and computer-aided detection, as well as three modes: diagnose mode, for reading direct digital mammography, computed radiography, and tomosynthesis; review mode, for technologists' management of clinical data and image display; and connect mode, for transmitting images and clinical data.
X-ray
In radiography, GE is launching a new mobile system, Optima XR240amx with FlashPad HD digital flat-panel detectors. The system was designed to be flexible enough to provide imaging in multiple clinical care environments, such as the emergency room or neonatal intensive care unit (NICU).
Providing high-resolution imaging is another major emphasis of the system, which supports 100-micron imaging at a detective quantum efficiency (DQE) of 75%. It supports wireless detectors in two sizes, 14 x 17-inch and 10 x 12-inch for NICU use. It also sports a small x-ray tube design that enables easy positioning under NICU incubators such as GE's Giraffe Carestation.
Meanwhile, Proteus XR/f is a new digital floor-mounted radiography room designed to be easy to site and use and to support imaging of all types of patients. The unit features an elevating table and cesium-iodide cassette-sized detectors.
Proteus XR/f's table supports patients up to 770 lb, and it can be lowered for below-the-knee exams or used for exams with the wireless detector untethered to image patients in wheelchairs and gurneys. A full charge on the detector provides up to 8.2 hours of use or 300 images, and the detector itself weighs only 5.7 lb. Proteus XR/f is not yet commercially available.
The last new x-ray offering in GE's booth is Precision 600FP, a classical radiography and fluoroscopy (R/F) system that uses 17 x 17-inch flat-panel cesium iodide digital detectors. The unit was designed to fit into existing space while supporting imaging of patients in wheelchairs and gurneys.
Proteus 600FP includes filters for reducing image noise and producing clearer images, as well as protocols for reducing radiation dose delivered to both patients and operators, such as allowing dose to be set at low, medium, or high. The system is not yet commercially available.
On the software side, GE is demonstrating software that will allow users of its x-ray systems to perform analysis on repeated and rejected x-ray studies. The software captures data from all x-ray systems installed at a facility, and it enables users to analyze exams by technologist, clinical procedure, and x-ray room. The tool is currently in beta testing and will be offered as part of GE's Healthcare Analytics Solutions offering.
Interventional x-ray
In interventional radiology, GE is showing its Discovery IGS 740, a laser-guided angiography system for interventional radiology and cardiology procedures that is designed to make 3D imaging easier to perform on patients in a variety of positions. GE is talking up how the system's bore size enables access to conebeam CT, which is increasingly being used in treatment planning and diagnosis.
The company is also pointing out advantages of its Assist software, which includes packages for a variety of clinical applications, from vascular surgery to liver embolization to radiofrequency ablation.
In other interventional news, the company announced that its Discovery IGS 730 system is now compatible with the Magnus operating table from the Maquet brand of Getinge Group. Combining the two products will allow hospitals to expand their range of surgical procedures and interventions and make better use of their hybrid operating rooms, according to GE.
Imaging informatics
GE has also announced the availability of its Centricity Imaging Collaboration Suite, a suite of distributed applications that enable care teams to view, manipulate, and share images throughout an enterprise. The suite is built on GE's Health Cloud and helps clinicians collaborate on patient cases, reduce CD handling costs, decrease repeat imaging, and reduce the amount of time spent preparing for multidisciplinary meetings.
GE also has new offerings for its Centricity Solutions for Enterprise Imaging software. The company has added an update to its Universal Viewer software through what GE calls integrated relevant clinical content (IRCC), which delivers relevant clinical information to users, such as lab data, radiology reports, patient history, and pathology data. IRCC also learns from radiologists' behavior to enable them to reach more confident diagnoses. The new version will begin shipping in January.
GE has also added an analytics package to its Enterprise Imaging Solutions portfolio that is designed to support the rapid deployment of analytics algorithms for x-ray, ultrasound, CT, MRI, cardiology, and enterprise imaging. The framework will run on GE Health Cloud, the company said.
 GE has developed a remote training program with InTouch Health.
GE has developed a remote training program with InTouch Health.Finally, GE is showing a remote training program the company has developed in collaboration with InTouch Health, an enterprise telehealth company. Called Virtual Onsite Training, the program includes what the companies call a remote presence device; a monitor on a mobile cart enables training personnel to interact with users over a video link with two-way interaction and communication. GE is rolling out the program after a successful two-year pilot implementation, the company said.
Molecular imaging
Discovery MI is a new PET/CT scanner first introduced at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) meeting in June 2016. Since that show, Discovery MI has received 510(k) clearance and two evaluation units have been shipped.
Among other features, GE is touting the scanner's use of LightBurst digital detectors, along with time-of-flight (TOF) and Q.Clear image reconstruction technology. GE believes the combination yields higher resolution and enables improved detection of small lesions.
Discovery MI also includes low-yield tracer capability with protocols that can lower dose by up to 50%, according to the company. GE said the new system's capabilities could also be used to support quantitative brain research. Discovery MI also utilizes GE's adaptive statistical iterative reconstruction-V (ASIR-V) techniques, as well as the firm's metal artifact reduction software.
The first Discovery MI units have shipped to Stanford University and Uppsala University Hospital in Sweden.