CHICAGO - Philips Medical Systems has brought a range of new products to the RSNA show floor, including a 64-slice CT scanner, a radiology information system, and a 1-tesla open superconducting MR scanner.
CT
Brilliance 64 is a new 64-slice CT scanner that Philips is launching at the RSNA show. The system features 40 mm of coverage per rotation, with images collected in a 64 x 0.625 matrix. A beta unit has been installed in Israel, with commercial shipments expected to begin in mid-2005, according to Jim Green, general manager of the vendor's CT business unit.
Philips is discussing the new functionality possible with the system, such as a combined exam that incorporates a lung scan in three seconds and a cardiac scan in seven seconds, or a whole-body study of a 1.5 m patient in 15 seconds. The scanner's fast-scanning capability makes it appropriate for a wide range of applications, from cardiac imaging to trauma to pediatrics, Green said.
Advanced applications include virtual colonoscopy software with a FiletView mode that provides a distinctive landscape view of the interior wall of the colon. The VC application started shipping in October with the release of version 2.0 of the company's Brilliance Extended Workspace workstation software. Philips' lung computer-aided detection (CAD) software started shipping in Europe earlier this year, and the company is expecting FDA clearance anytime.
The company is also demonstrating Brilliance Big Bore 16, a wide-bore CT scanner with an 85-cm diameter bore and a 60-cm field-of-view. The system has been upgraded to 16 slices after previously being available in a single-slice version. Although it was designed originally for radiation therapy applications, Philips is finding that many sites are choosing the system for other uses in which a wide bore is helpful, such as interventional applications.
MRI
Taking center stage in the MRI section of Philips' booth is Panorama 1.0T, a 1-tesla open superconducting scanner. The first system has been installed at an imaging center in Las Vegas, and commercial shipments are expected to begin in the second quarter of 2005.
Philips is positioning the system as a general-purpose scanner that can handle a wide variety of advanced applications, rather than as system dedicated to interventional applications or claustrophobic patients. The magnet's vertical-field design gives it image quality that is close to that of a 1.5-tesla scanner, according to Guido Stomp, global field marketing director for MR.
Panorama 1.0T is actively shielded and installs in the same space as a conventional 1.5-tesla scanner, and also features a patient table that's motorized to enable it to move laterally in front of the scanner. This enables users to keep the region of interest that's being scanned directly in the isocenter of the system's magnetic field, Stomp said. This can be particularly useful for orthopedic extremity applications, such as scanning hands and feet.
Philips is also showing new Quasar Dual gradients for its 3-tesla Intera Achieva scanner, with an amplitude of 80 mT/m and a slew rate of 200 T/m/sec. Work-in-progress developments being highlighted include k-Blast, an ultra-fast imaging protocol for cardiac and abdominal imaging.
For the Panorama 0.6T and Panorama 0.23T permanent magnet open systems, the company is launching new release 6.0 software that includes applications like echo-planar diffusion-weighed imaging.
Finally, Philips is unveiling its NetForum concept at the show. NetForum is a special area of the Philips Web site where customers can interact with each other and download ExamCards, which are sets of imaging protocols for Philips MR scanners.
PACS/RIS
Xtenity RIS is making its debut. Xtenity is based on a RIS contributed by partner Epic Systems, but adds the vendor's Vequion user interface and other enhancements, said Daryl Tom, vice president of medical IT, North America.
It can integrate with the vendor's EasyAccess PACS network, imaging modalities, workstations, and other IT systems, Tom said. Features include a rules-based scheduling engine, a tool set that provides clinical documentation templates and shortcuts, automated charge capture for both referring clinicians and radiologists, as well as integrated speech recognition capability.
Xtenity will be available in the third quarter of 2005, and Philips will begin taking orders in the second quarter next year, Tom said. At next year's Healthcare Information and Management Systems Society (HIMSS) meeting, Philips will debut an Xtenity HIS product line, which will also be powered by Epic.
Philips is also demonstrating EasyRIS, a RIS targeted at the European and Asian markets.
In PACS developments, Philips is rolling out release 10 for its EasyAccess line. New features include user concurrency, lossless compression for its RAID archive, orthopedic templates, and an e-learning tool. The firm has also incorporated its ViewForum plug-in, yielding capabilities such as 3D/MPR and coronary 3D.
Other release 10 enhancements include the addition of JPEG 2000 compression for its Web distribution system and desktop integration with any RIS vendor.
Philips is also providing details on EasyAccess Focus, a PACS targeted for low-volume facilities, as well as PACS financing options available via Philips division Philips Capital. The firm has also added workflow analysis and benchmark studies to its professional services portfolio.
Also on display are the vendor's EasyVision Mammo digital mammography workstation and the latest release of its cardiac PACS network.
Molecular imaging
Philips has upgraded its Gemini hybrid PET/CT platform with Gemini GXL, which uses new detector materials and upgraded electronics to double the system's performance, according to Ian Farmer, senior vice president and general manager of the company's nuclear medicine unit.
Philips boosted Gemini GXL's performance by "doping" the system's gadolinium orthosilicate (GSO) scintillation crystals with zirconium. As a result, the count rate of the new GSO-Zr detector material has improved to 70 kcps noise equivalent counts (NEC) from 33 kcps previously, Farmer said. Philips has also increased the length of the crystals, from 20 mm to 30 mm, enabling the system to collect more data.
Other enhancements on the Gemini GXL include high-speed electronics and a new 3D reconstruction mode. Meanwhile, a new line of response (LOR) algorithm measures each scintillation event individually, instead of averaging a large group of events, resulting in better accuracy. Philips added two new CPUs to Gemini GXL to handle the additional data load, Farmer said. Finally, Philips expanded the diameter of the PET bore in the system from 63 mm to 70 mm, matching the diameter of the CT bore used in the system.
Beta versions of Gemini GXL will begin shipping in February, with commercial production scheduled for May. The system comes in versions with six-, 10-, and 16-slice CT versions.
In new SPECT developments, Philips announced new electronics for its Precedence SPECT/CT systems, which come in six- and 16-slice configurations. First announced at the Society of Nuclear Medicine meeting in June, Precedence now has higher speed and more accurate electronics, according to Farmer, with spatial resolution improving from 7 mm previously to 5.1 mm now. The system's detector's are now energy-independent, and can conduct studies of radiopharmaceuticals with different energies in a single pass.
Philips has also expanded the number of applications available with its JetStream nuclear medicine computer. JetStream originally launched with only cardiology applications, but the computer has now been enhanced to support all nuclear medicine applications.
Ultrasound
Philips is spotlighting iU22, its premium ultrasound scanner that debuted earlier this year. iU22 offers capabilities such as real-time 4D imaging, voice-activated control and annotation, and automated image optimization technologies, said Patricia Venters, marketing director for general imaging ultrasound, North America.
iU22 can handle 4D ob/gyn and breast examinations, and can also be deployed for abdominal, musculoskeletal, pediatrics, small parts, and vascular applications. The company is emphasizing the ergonomic capabilities of iU22, including a fully articulating control panel, lighter transducer cables, and angled, easy-access transducer connectors.
iU22 also makes use of the vendor's xStream architecture, which yields speedy acquisition, manipulation, and quantification of full-volume datasets, Philips said. Other features include 3D and 4D imaging, as well as multiplanar reconstruction revolution that rivals acquired 2D plane resolution, according to the firm.
Over 500 iU22 systems have been installed in North America, Venters said.
Although it's not showing the model on the show floor, Philips is also discussing iE33, the latest addition to its echocardiography portfolio. The premium unit features fully integrated 2D and 3D cardiac quantification software for measurements such as left ventricle volume and ejection fraction.
iE33 can generate 3D measurements of the heart in less than a minute. Philips has also incorporated 2D image quality enhancements and its Live 3D imaging technique, as well as ergonomic improvements, voice-activated controls, and automated image optimization technologies.
Philips' QLAB quantification software employs 3D border detection to provide rapid access to ventricular volumes of the whole heart and waveforms that simultaneously show the function of 17 different heart segments, according to the company.
The system's transducers include S5-1 with the vendor's PureWave crystal technology, which utilizes a class of piezoelectric material Philips says allows for greater transmit frequency than conventional transducers. The company has also included its xMatrix transducer in the technology in its X3-1 probe, yielding real-time 3D volumes and simultaneous views of the heart from the same heartbeat.
X-ray
DigitalDiagnost VM is a new one-detector radiography room that uses a flat-panel detector in a semirobotic rolling wall stand. The unit can reduce radiation dose by 50% and throughput from 25 minutes per exam to 10-15 minutes, according to the company. The first installation of the system was in September.
Other x-ray systems being highlighted in the company's booth are the MultiDiagnost Eleva radiography/fluoroscopy system, which is being shown with an FD20 flat-panel detector as a work-in-progress. OmniDiagnost Eleva is a combined DR/CR unit, while EasyDiagnost Eleva is a conventional R/F unit.
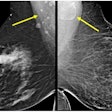
Philips is entering the market for full-field digital mammography (FFDM) systems with MammoDiagnost FD Eleva, being shown as a work-in-progress. The unit is a slot-scanning system with 50-micron resolution in screening mode and 25-micron resolution in diagnostic mode. Philips is already shipping the unit in Europe, and will introduce it in the U.S. after FDA approval.
In vascular imaging, Allura XPer FD 20 uses a flat-panel detector with a 2K x 2K image acquisition and storage capability. The system has a new user interface, and Philips is demonstrating a technique for acquiring CT-like images using the system's rotation and the company's 3D RA tool. Shipments of the system started in September.
Finally, Philips demonstrated new enhancements to its Ambient Experience concept, which uses technology from other Philips business units to make the imaging process more patient-friendly. The first Ambient Experience radiology suite has been installed at Advocate Lutheran General Children's Hospital in the Chicago area, according to the company.
By Brian Casey and Erik L. Ridley
AuntMinnie.com staff writers
November 30, 2004
Copyright © 2004 AuntMinnie.com