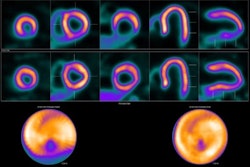
GE HealthCare has received U.S. Food and Drug Administration (FDA) approval for its flurpiridaz F-18 radiotracer for PET imaging of coronary artery disease.
Flurpiridaz F-18 (Flyrcado) is indicated for patients with known or suspected coronary artery disease (CAD), with studies showing the radiotracer delivers higher diagnostic efficacy compared to SPECT myocardial perfusion imaging (MPI), GE HealthCare said.
Flurpiridaz F-18 can be manufactured in an offsite pharmacy and delivered as a ready-to-use unit dose, the company noted. The radiotracer has a half-life of 109 minutes, which is significantly longer than existing PET MPI tracers, GE HealthCare added.
“Although PET MPI as a modality enables high diagnostic accuracy as compared with SPECT MPI, only a minority of annual PET scans in the U.S. are PET MPI because of limited access to the currently available PET tracers,” noted Jamshid Maddahi, MD, of the University of California, Los Angeles, who led clinical trials for the imaging agent, in a news release.
CAD is the most common form of heart disease and remains the leading cause of death for men and women in the U.S., with 695,000 deaths recorded in 20214. During trials, flurpiridaz F 18 was compared with both invasive coronary angiography as a standard of truth to determine diagnostic efficacy in detecting CAD, as well as with SPECT MPI.
GE HealthCare acquired exclusive global commercialization rights for flurpiridaz F-18 from Lantheus in 2017 and has led the funding and development of the product through to approval. Lantheus collaborated on the development and will also collaborate on commercialization through a joint steering committee. Lantheus is entitled to royalties based on commercial sales milestones, according to the release.
Flyrcado will be available in initial U.S. markets in early 2025 with expanding availability thereafter, GE HealthCare said.