PET scans have demonstrated for the first time that only women appear to experience specific cellular neuroinflammation related to aging in the brain's hypothalamus, a process that may be related to menopause, according to a study published on August 3 in Scientific Reports.
Researchers at Weill Cornell Medicine in New York City analyzed PET scans performed with a radiotracer that detected translocator protein (TSPO) activity in hypothalamus glial cells in a group of healthy men and women. They found that only women showed age-correlated hypothalamic TSPO expression.
"We suggest this novel result is relevant to understanding a stark sex difference in human aging: that only women undergo loss of fertility -- menopause -- at mid-life," wrote corresponding author Dr. Tracy Butler, an associate professor of neurology in radiology, and colleagues.
The hypothalamus is best known as the controller of hormone secretion to regulate essential bodily functions, such as reproduction. Recent work in animal models suggests that the hypothalamus serves as a hub for cellular activity involved in neural inflammation and may mediate age-related brain and body changes, such as those that occur in menopause, according to the authors.
To understand how these findings translate to humans, the researchers assessed the contribution of age and sex to hypothalamic TSPO expression in a group of healthy subjects (n = 43, 19 female, ages 23 to 78). Participants underwent TSPO-PET scans (Biograph PET/CT, Siemens Healthineers) at Weill Cornell Medicine between 2013 and 2019 during studies investigating epilepsy, hypertension, chronic fatigue syndrome, Parkinson's disease, and Gulf War syndrome.
To assess the contribution of age and sex to hypothalamic TSPO expression among the group, researchers measured the binding potential of a radiotracer (carbon-11 PK11195) designed to reveal TSPO expressed on the outer membrane of activated glial cells.
In the combined group of women and men, the authors found robust age-correlated TSPO expression in the thalamus, but not the hypothalamus. However, when examining TSPO-PET results in women and men separately, they found that only women showed age-correlated hypothalamic TSPO expression (R = 0.49, p = 0.032).
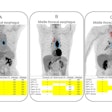
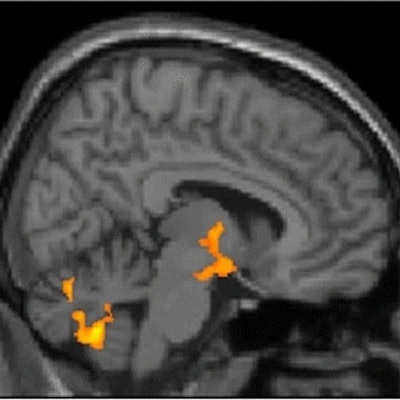
![A sagittal view (right of midline; X = 8) of whole-brain statistical z-map showing regions where brain age-correlated TSPO expression was greater in women than men, with prominent results in a region spanning both the hypothalamus and thalamus (hypothalamus peak: X = 11, Y = -7, Z = - 13 [Z = 4.2]; thalamus peak: X = 4, Y = -6, Z = 0 [Z =5.1]; center of gravity in the hypothalamus: X = 10, Y = -5, Z = -9. This sex difference corresponds to significant age-correlated TSPO expression in the hypothalamus: X = 10, Y = -5, Z = -9. This sex difference corresponds to significant age-correlated TSPO expression in the hypothalamus in women (peak: X = 8, Y = 0, Z = -12 [t = 3.3; p-corrected = 0.002]), but not men. There were no areas of greater age-correlated TSPO expression in men as compared to women. Image courtesy of Scientific Reports.](https://img.auntminnie.com/files/base/smg/all/image/2022/08/am.2022_08_08_22_09_9172_2022_08_09_TSPO_women_resized.png?auto=format%2Ccompress&fit=max&q=70&w=400) A sagittal view (right of midline; X = 8) of whole-brain statistical z-map showing regions where brain age-correlated TSPO expression was greater in women than men, with prominent results in a region spanning both the hypothalamus and thalamus (hypothalamus peak: X = 11, Y = -7, Z = - 13 [Z = 4.2]; thalamus peak: X = 4, Y = -6, Z = 0 [Z =5.1]; center of gravity in the hypothalamus: X = 10, Y = -5, Z = -9. This sex difference corresponds to significant age-correlated TSPO expression in the hypothalamus: X = 10, Y = -5, Z = -9. This sex difference corresponds to significant age-correlated TSPO expression in the hypothalamus in women (peak: X = 8, Y = 0, Z = -12 [t = 3.3; p-corrected = 0.002]), but not men. There were no areas of greater age-correlated TSPO expression in men as compared to women. Image courtesy of Scientific Reports.
A sagittal view (right of midline; X = 8) of whole-brain statistical z-map showing regions where brain age-correlated TSPO expression was greater in women than men, with prominent results in a region spanning both the hypothalamus and thalamus (hypothalamus peak: X = 11, Y = -7, Z = - 13 [Z = 4.2]; thalamus peak: X = 4, Y = -6, Z = 0 [Z =5.1]; center of gravity in the hypothalamus: X = 10, Y = -5, Z = -9. This sex difference corresponds to significant age-correlated TSPO expression in the hypothalamus: X = 10, Y = -5, Z = -9. This sex difference corresponds to significant age-correlated TSPO expression in the hypothalamus in women (peak: X = 8, Y = 0, Z = -12 [t = 3.3; p-corrected = 0.002]), but not men. There were no areas of greater age-correlated TSPO expression in men as compared to women. Image courtesy of Scientific Reports."This first assessment of hypothalamic microglial activation in humans demonstrates important age and sex effects," the authors stated.
Ultimately, menopause is associated with a markedly increased risk of cognitive decline and age-related disease, including Alzheimer's disease and cardiovascular disease, the authors wrote. In rodent models of menopause, estrogen supplementation protects against many of these changes, while in humans, the benefits of postmenopausal hormonal replacement therapy remain uncertain despite decades of study.
The finding in this study of age-correlated hypothalamic inflammation in women could have implications for understanding and perhaps altering reproductive aging in women, the authors wrote.
Future studies should include longitudinal study design, careful assessment of subject hormonal and reproductive status, and neuroimage acquisition and processing techniques optimized for assessing small structures such as the hypothalamus, the group added.
"Understanding species and sex differences in aging is essential to the development of effective therapies for age-related diseases, and perhaps aging itself," Butler and colleagues concluded.