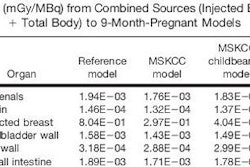
Interventional device developer Abbott Vascular of Redwood City, CA, is touting early clinical results from a new trial evaluating the safety and performance of a new class of bioabsorbable drug-eluting stents.
Results from the ABSORB clinical trial are being presented this week at the Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium in Washington, DC. Initial results in the trial for the first 30 patients indicated no major adverse cardiac events and no stent thrombosis at 30 days for patients who received Abbott's bioabsorbable everolimus-eluting stent.
The ABSORB trial is a prospective, nonrandomized study and has been designed to enroll up to 60 patients in Belgium, Denmark, France, New Zealand, Poland, and the Netherlands. The key end points of the study include an assessment of safety at 30, 180, and 270 days, with an annual follow-up for up to five years, and successful deployment of the bioabsorbable drug-eluting stent.
By AuntMinnie.com staff writers
October 25, 2006
Related Reading
Abbott launches Xience V stent, October 3, 2006
Abbott nets CE Mark for Emboshield Pro, September 13, 2006
Abbott completes Guidant vascular unit purchase, April 21, 2006
Abbott debuts catheter for chronic occlusions, February 27, 2006
Abbott amends Boston Scientific agreement, January 17, 2006
Copyright © 2006 AuntMinnie.com