MRI contrast developer Epix Pharmaceuticals has submitted a formal appeal to the Center for Drug Evaluation and Research (CDER) at the U.S. Food and Drug Administration (FDA), asking the CDER to approve its blood-pool imaging agent Vasovist (gadofosveset trisodium injection).
In the filing, Epix asked that an advisory committee be considered as part of the appeal process. The Lexington, MA-based company expects the CDER to respond to its submission within approximately 30 days, although a decision could be postponed if CDER grants the advisory committee meeting.
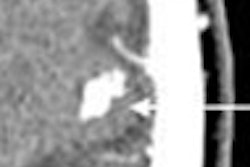
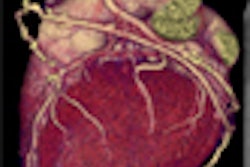
Vasovist is an injectable intravascular contrast agent designed to provide visual imaging of the vascular system through MR angiography. The initial target indication for Vasovist is for use in MR angiography imaging of noncoronary vascular disease. Vasovist has been approved for marketing in 30 countries outside of the U.S.
By AuntMinnie.com staff writers
February 28, 2007
Related Reading
Epix to restate past financial reports, February 26, 2007
Epix files U.S. Vasovist appeal, December 15, 2006
Epix releases clinical trial results for blood-clot agent, November 30, 2006
Epix nets Canadian OK for Vasovist, November 6, 2006
Epix nets Australian approval, September 20, 2006
Copyright © 2007 AuntMinnie.com