Bronchopulmonary Dysplasia/Chronic Lung Disease of Prematurity:
View cases of BDP
Clinical:
Premature infants often have a surfactant deficiency- those who
fail to respond to initial therapy often require prolonged
mechanical ventilation and follow a typical clinical course.
Bronchopulmonary dysplasia (BPD), now referred to as chronic lung
disease of prematurity, follows prolonged respirator therapy and
occurs secondary to the toxic effects of high oxygen
concentrations, as well as barotrauma (interstitial emphysema,
airway smooth muscle hypertrophy, epithelial squamous metaplasia,
and peribronchial fibrosis)- essentially, it is the result of
neonatal lung injury with slow resolution [1,4].
The incidence of BPD is difficult to determine. Probably less
than 20% of premature infants who weigh less than 1500 grams at
birth will develop BPD., while about 30% weighing less than 1000
gm will develop the condition [3]. Optimal treatment for BPD is
the prevention of hyaline membrane disease. BPD has varying
severity, but can be associated with significant early morbidity
and mortality. The requirement for supplemental oxygen at 1 month
of age has been shown to be a poor predictor of eventual outcome
[1]. About 30% of affected neonates will have normal a CXR by age
3 years, and less than 10% have residual clinical or radiographic
evidence of abnormal pulmonary function. However, up to 30% will
have some form of neurodevelopmental handicap.
Given modern day ventilator strategies, premature infants now
receive prenatal corticosteroids, will be ventilated for shorter
periods of time, and with new ventilator settings, resulting in
less-pronounced alveolar septal fibrosis and subtler imaging
findings [6].
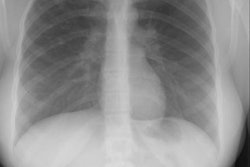
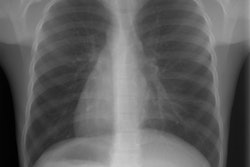
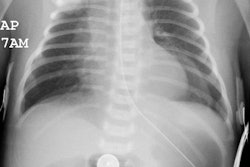
There are four distinct radiographic stages to BPD:
- Stage I: Day 1-4. Characterized by reticulogranularity (indistinguishable from respiratory distress syndrome) with air bronchograms. Pathologically there is bronchiolar mucosal necrosis and the air spaces are filled with fibrin, red cells, and desquamated pneumocytes.
- Stage II: Day 4-10. "White out" of the lung fields. CHF/pulmonary edema may complicate this stage if the ductus reopens. Pathologically there is exudative necrosis of the alveolar epithelium.
- Stage III: Day 10-30. Bubbly, rounded lucencies, with coarse lung markings appear on the CXR. Migrating atelectasis is a common feature of all stages of BPD. Pathologically the alveoli are over-distended due to small airway plugging with scarred lung, and widespread bronchial and bronchiolar mucosal metaplasia.
- Stage IV: After 30 days. Almost radiographically identical to stage III, but may have increasing fibrosis and enlarged cystic changes.
In Wilson-Mikity Syndrome the changes are identical to
bronchopulmonary dysplasia, but the affected infants have never
received respirator therapy. In these patients respiratory
distress usually begins toward the end of the first week of life.
Changes in the treatment of surfactant deficiency syndrome with
adaptation of low-pressure and oscillatory ventilation techniques,
lower concentrations of supplemental oxygen, along with antenatal
steroid administration and post natal exogenous surfactant, have
reduced the incidence of classic BPD [4,5]. However, the increased
survival of extremely premature (24-26 week gestation infants) has
resulted in the emergence of a new type of BPD with less airway
disease and less fibrosis, but histologic features of arrested
lung development [4].
X-ray:
CXR will demonstrate coarsened lung markings/interstitial
thickening, focal areas of overinflation or generalized
overinflation, cystic areas of lucency, and bands of
atelectasis/scarring.
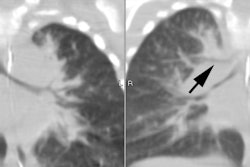
CT findings in BPD survivors include areas of reduced lung attenuation and perfusion (mosaic appearance), bronchial wall thickening, linear opacities, and bullae [2].
REFERENCES:
(1) J Thorac Imag 1986; Williams JL, et al. Bronchopulmonary dysplasia. 1 (4): 16-24
(2) AJR 2000; Howling SJ, et al. Pulmonary sequelae of bronchopulmonary dysplasia survivors: High-resolution CT findings. 174: 1323-1326
(3) Radiographics 2005; Agrons G, et al. Lung disease in
premature neonates: radiologic-pathologic correlation. 25:
1047-1073
(4) Radiographics 2017; Semple TR, et al. Interstitial lung
disease in children made easier...well, almost. 37: 1679-1703
(5) AJR 2018; Liszewski MC, Lee EY. Neonatal lung disorders:
pattern re to diagnosis. 210: 964-975
(6) AJR 2019; Liang T, et al. Childhood interstitial (diffuse)
lung disease: pattern recognition approach to diagnosis in
infants. 212: 958-967