Imaging software developer EDDA Technology of Princeton, NJ, has received Food and Drug Administration clearance for its IQQA-Chest software for analysis of digital radiography chest images.
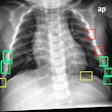
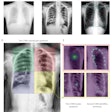
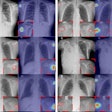
IQQA-Chest is designed for both the identification and quantification of lung nodules on digital projection chest radiography. The software has a lesion-specific image-enhancement viewing (LSEV) mode that dynamically details and highlights suspicious areas that could be nodular abnormalities. Once suspicious nodules are identified, the software offers regional analysis tools for the quantification of lesion characteristics, including size, density, and shape.
IQQA-Chest automatically assembles diagnostic information into a clinical report for referring physicians and for follow-up review. The software supports the DICOM 3.0 standard, and runs on a standard PC platform, according to the company.
By AuntMinnie.com staff writers
November 22, 2004
Copyright © 2004 AuntMinnie.com