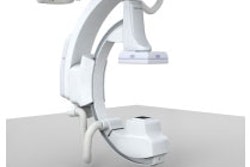
Multimodality vendor Siemens Medical Solutions of Malvern, PA, has received U.S. Food and Drug Administration 510(k) clearance for a pair of new technologies for its Axiom vascular imaging systems.
The company received the clearances for wireless footswitch and voice control enhancements for the Axiom line. Siemens believes the wireless footswitch eliminates safety hazards caused by cables in the imaging suite, while the voice control option enables physicians to stay focused on patients rather than searching for buttons on equipment.
Siemens made the announcement in conjunction with this week's Society of Interventional Radiology (SIR) meeting in Toronto.
By AuntMinnie.com staff writers
March 30, 2006
Related Reading
Siemens to unveil Inveon, 40-slice PET/CT, at AMI, March 24, 2006
Siemens debuts new ultrasound products at AIUM, March 23, 2006
Siemens grants Biosense Webster AcuNav distribution, March 17, 2006
Siemens adds hybrid viewer, new Soarian package, March 14, 2006
Siemens, Cleveland Clinic ink SPECT tech deal, March 9, 2006
Copyright © 2006 AuntMinnie.com