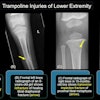
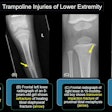
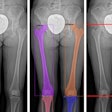
GE Medical Systems has received Food and Drug Administration clearance to market its Revolution XR/d dual-detector digital x-ray system for dual-energy subtraction studies. The dual-energy subtraction application allows radiologists to take two successive x-ray images of the chest in less than a second, according to the Waukesha, WI-based vendor.
The system then automatically generates a standard radiograph, an image of the soft tissue with the bones removed, and an image of the skeletal system. GE believes this set of images will allow radiologists to detect smaller pathology and reduce the need for additional scanning procedures.
Physicians at the University of Chicago Hospital and Charité University Hospital in Berlin, Germany, have been using the application in preclinical trials, according to GE. The vendor has more than 400 flat-panel x-ray systems currently installed in radiology, mammography, and cardiology departments.
By AuntMinnie.com staff writers
September 5, 2001
Related Reading
GE digital x-ray sales surge in Q2, July 12, 2001
Copyright © 2001 AuntMinnie.com