Pleuropulmonary Blastoma:
Clinical:
Pleuropulmonary blastoma is the most common primary lung malignancy in children, but is overall a very rare neoplasm representing only 0.25-0.50% of all primary lung malignancies [5]. The lesion is an embryonal or blastemic neoplasm found in children and extensive chest wall involvement is usually seen. The tumor is similar to the adult pulmonary blastoma (in that it contains both mesenchymal sarcomatous and blastemal elements], but lacks an epithelial component.Patients present with respiratory distress in most cases and over 75% of cases occur in children under the age of 5-6 years. Other symptoms include fever, cough, pneumothorax, anorexia, and malaise. The lesion is more common in whites. Over 70% of the lesions are right sided and a family history of pleuropulmonary blastoma or other childhood neoplasms is found in over 25% of cases. Approximately two-thirds of children with a PPB have a heterozygous germline mutation in the DICER1 gene located on chromosome 14q32.12[4]. DICER1 is a microRNA processing gene that is critical as both a developmental gene and a tumor suppressor gene, regulating over 30% of protein-coding genes at the post-transcriptional level [4]. A DICER1 mutation demonstrates an autosomal dominant inheritance pattern with moderate penetrance [5]. DICER1 PPB syndrome is found in about 10% of patients with a PPB (although other authors indicate that 65-70% of children with PPB have a heterozygous mutation in DICER1 [5]) and several other non-pulmonary neoplasms have been recognized in this syndrome- most commonly cystic nephroma, but also reported are thyroid adenoma or thyroid cancer, nasal chondromesenchymal hamartoma, ciliary body medulloepithelioma, pineoblastoma, pituitary blastoma, embryonal rhabdomyosarcoma of the uterine cervix, and sex cord stromal tumors of the ovary [4,5].
Most lesions arise in the lung periphery, but in 15-25% of the cases, the mass is extrapleural with an attachment to the parietal pleura or with involvement of the diaphragm or mediastinum.
Lesions are classified as type I, II, or III [5]. Type I lesions (least common- median age at diagnosis is 10 month) are exclusively cystic without a grossly detectable solid component. Type II lesions exhibit both cystic and solid components, and type III lesions are solid tumors [4,5]. The age at presentation is related to the tumor type- with type I lesion presenting in younger children (mean 10 months), type II (mean 34 months), and type III in older children (44 months) [4]. The lesions types represent a continuum with initial lesion is felt to be type I that subsequently progresses [3].
Treatment is surgical excision if possible, with adjuvant chemotherapy with or without XRT for type II and III lesions [5]. Prognosis is still poor as the tumor tends to recur locally- especially type II and type III lesions [3]. Metastatic disease occurs in approximately 30% of type II and type III lesions [5]. Metastasis to the lungs or CNS can be seen. Five year survival is strongly correlated to the tumor pathology- 83% to over 90% for type I lesions and 49% for type II, and 42% for III lesions even with multimodality treatment [3,4,5].
X-ray:
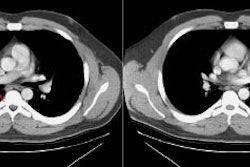
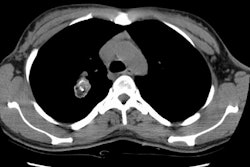
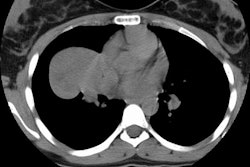
Type I lesions may manifest as a single or a multi-cystic lesion that is often air filled [5]. The differential would include a type I or type II CCAM or a pneumatocele [5]. Type I PPB and Type I CCAM are indistinguishable at imaging, but CCAMs are often discovered antenatally during the second trimester, whereas PPB is more often discovered postnatally [5].Type II lesions show air- or fluid-filled cavities with possible air fluid levels along with solid internal nodules [5]. Type III lesions are solid that show low attenuation and homogeneous or heterogeneous enhancement after contrast administration [5] (CT can demonstrate a large, heterogeneous, low attenuation mass which contains whorls of soft tissue).
The lesion may appear as a consolidation or opacification of a hemithorax mimicking a pneumonia or empyema. The lesion usually has extensive necrosis and may have a air-filled multicystic appearance. Sonography of the thorax can also confirm a multicystic appearance.
Pleural effusion is commonly seen in association with the mass (often grossly bloddy on analysis). Pneumothorax may also occur.
REFERENCES:
(1) AJR 1996; Dobard GF, et al. Pleuropulmonary blastoma with vascular invasion. 166(2): 308 (No abstract available)
(2) J Thorac Imag 1995, 10: p. 112-116
(3) Cancer 1997; Priest JR, et al.
Pleuropulmonary blastoma: a clinicopathologic study of 50 cases.
80: 147-161
(4) Radiology 2014; Kousari YM, et al. Case 211: pleuropulmonary
blastoma in association with cystic nephroma- DICER1 syndrome.
273: 622-625
(5) Radiographics 2018; Lichtenberger JP, et al. Primary lung tumors in children: radiologic-pathologic correlation. 38: 2151-2172