Computer-aided detection (CAD) technology may soon be deployed for lung cancer screening, if a Maryland startup has its way. Rather than joining other CAD firms in developing breast cancer screening devices, Deus Technologies has submitted a premarket approval application (PMA) to the Food and Drug Administration for its RapidScreen RS-2000 lung cancer CAD unit.
Although there are 160,000 lung cancer deaths in the U.S. each year, few clinicians do annual chest x-rays to screen for the disease, according to Deus president and CEO Michael Yeh, Ph.D. Instead, most perform x-rays only when symptoms of disease manifest themselves. For a high-risk population, lung cancer screening with chest x-rays and CAD technology could be an effective way for catching early-stage cancers that is cheaper than CT.
Deus hopes to receive FDA clearance by the end of April for RapidScreen, which marks areas on a chest x-ray that may have features associated with early-stage lung cancer.
"In the past, clinicians didn’t necessarily consider chest x-ray for lung cancer screening," Yeh said. "But with CAD technology, it is an effective and affordable option."
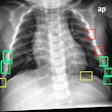
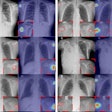
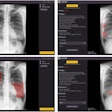
Like mammography CAD units, RapidScreen digitizes analog x-ray films that have been loaded into a scanner. The digital image is then analyzed with RapidScreen’s pattern recognition algorithms, checking for any of 87 features associated with pulmonary nodules and circling areas of interest on both the soft display and the printed image. The device searches for nodules with a diameter smaller than 30 mm.
"With mammography screening, a doctor knows what to look for, and early-stage cancers are detected 75% to 80% of the time," Yeh said. "In a typical clinical setup for chest x-ray, a doctor spends about 30 seconds (reviewing an image), and doesn’t necessarily look for lung cancer, particularly stage I cancer, but rather for TB or other diseases. The percentage of missed early-stage lung cancers is very high -- only about 15% of detected lung cancers are early-stage."
Rockville, MD-based Deus emerged from government contracts firm Caelum Research, which Yeh, a meteorologist, established in 1987. Caelum offered R&D support in the areas of satellite remote sensing and pattern recognition, and Yeh wanted to apply this knowledge to medical applications.
With the help of some physicians at Georgetown Medical Center in Washington, DC, and funding from the National Institutes of Health, Yeh began to pursue the use of CAD technology to identify early-stage lung cancer. In 1998, Deus Technologies spun off from Caelum to become the R&D center for RapidScreen.
The company first contacted the FDA three years ago. At the time, the agency directed Deus to prepare a PMA for RapidScreen. "At our first meeting with the FDA, the agency told us clearly that our application could not be a 510(k), since the device would influence doctors’ diagnoses," Yeh said.
Deus conducted a pilot study with RapidScreen in 1999, and the FDA approved the study’s protocol for a clinical trial, which concluded in July 2000. The double-blinded trial found that RapidScreen could improve detection of lesions 9 mm to 14 mm by 24%, and lesions 15 mm to 19 mm by 20%, resulting in an overall increase from 65% to 84% of lesions detected.
Deus would like to establish partnerships with a few large manufacturers to market RapidScreen, but also plans to sell the unit directly, Yeh said. The company is also exploring the possibility of leasing the units to clinics or hospitals and charging per procedure.
Deus estimates that a RapidScreen procedure will add $15 to $30 to the cost of a chest exam, and expects that upon clearance by the FDA, the Health Care Financing Administration will add lung cancer CAD procedures to its reimbursement coding. The company is in negotiations with two Japanese companies for RapidScreen marketing rights in that country, and has also begun discussions with the state of Maryland, which is considering the establishment of a lung cancer screening program with tobacco-settlement funding.
In the R&D pipeline for RapidScreen is a unit designed for images acquired with digital radiography systems, as well as expanded capabilities for breast cancer and tuberculosis screening. Deus also plans to develop RapidScreen for use with other imaging methods. The company has completed a software package called RapidDisplay, which helps doctors match films between CT exams, measure the size of a particular nodule, and predict the time it will take the nodule to double in size.
"We’re expanding in two different directions," Yeh said. "We’d like to add more applications to our technology, like breast cancer and tuberculosis, but we’d also like to develop RapidScreen for use with new modalities."
By Kate Madden Yee
AuntMinnie.com contributing writer
April 20, 2001
Related Reading
Deus nears approval for lung cancer detection unit, March 27, 2001
FDG-PET turns in high sensitivity for small- and non-small cell lung cancers, November 9, 2000
Volumetrically determined growth rate, October 23, 2000
PET outperforms CT in detecting non-small-cell lung lesions, July 26, 2000
New CAD scheme for lung nodules reduces false positives, July 4, 2000
CT and SPECT explored to better classify lung nodules, January 28, 2000
Click here to post your comments about this story. Please include the headline of the article in your message.
Copyright © 2001 AuntMinnie.com