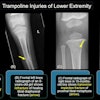
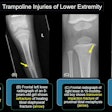
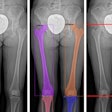
X-ray developer Lodox Systems has received U.S. Food and Drug Administration 510(k) clearance to market its charge-coupled device (CCD) full-body x-ray system. The company will be establishing offices in the U.S. to bring the product to market, according to Herman Potgieter, CEO of the Sandton, South Africa-based manufacturer.
By AuntMinnie.com staff writersAugust 8, 2002
Related Reading
Lodox debuts at RSNA show with new CCD-based digital x-ray system, November 25, 2001
Copyright © 2002 AuntMinnie.com