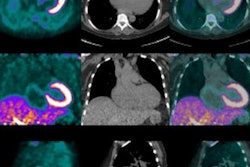
Preliminary study results indicate that F-18 florbetapir (Amyvid) can detect cardiac amyloidosis, a buildup of protein that can lead to various forms of heart disease. The results hint that Amyvid could be useful beyond its core indication of detecting beta-amyloid deposits in the brain.
Systemic amyloidosis is a disorder characterized by deposits of insoluble, abnormal protein fibers that impair tissue structure and function. The condition can produce abnormal fragments of immunoglobulin light-chain protein and transthyretin protein, noted lead author Dr. Karen Wells, assistant professor in the department of medicine at the University of Tennessee Graduate School of Medicine.
There are more than 80 different mutations of the transthyretin gene, which are responsible for hereditary forms of cardiac amyloid. In addition, the transthyretin protein in its nonmutated form can cause cardiac amyloid deposits of more than 10% in men ages 65 years and older, with increasing frequency as men age. In turn, cardiac amyloidosis can result in arrhythmia, restrictive cardiomyopathy, and progressive heart failure.
The diagnostic approach to cardiac amyloidosis is primarily to use nonspecific imaging such as electrocardiograms, 2D echocardiograms, cardiac MRI, and planar scintigraphy with metaiodobenzylguanidine (MIBG). More specific diagnoses can be obtained with endomyocardial biopsy, Wells added, but the procedure is invasive.
The current study sought to investigate whether amyloid deposits can be noninvasively visualized and quantified in patients with transthyretin and systemic light-chain cardiac amyloidosis. The researchers compared the results with myocardial florbetapir uptake in healthy control subjects. Wells presented the study at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) annual meeting.
The study included eight patients with a mean age of 66.7 years (range, 58 to 74 years) and a diagnosis of cardiac amyloidosis, along with three healthy controls. The imaging protocol consisted of a low-dose CT scan, followed by a 30-minute, cardiac-gated dynamic PET scan (Biograph mCT, Siemens Healthcare) with simultaneous injection of 370 MBq of florbetapir.
Florbetapir is currently marketed under the trade name Amyvid in the U.S. and Europe by Eli Lilly and its subsidiary Avid Radiopharmaceuticals. It has been cleared for use in imaging beta-amyloid deposits with PET in adults being assessed for Alzheimer's disease or other causes of cognitive issues.
The researchers generated time activity curves (TAC) from the regions of interest over the left ventricular myocardium to measure standardized uptake values (SUV) for florbetapir, and they visually interpreted the resulting dynamic cardiac and whole-body PET/CT images.
All eight patients had cardiac amyloidosis determined by 2D echocardiography; four were also confirmed by endomyocardial biopsy and the other four were confirmed by cardiac MRI with extracardiac tissue biopsy. Three patients were found to have mutated forms of transthyretin amyloid, while the other five had light-chain amyloidosis.
TAC results
The time activity curves yielded what Wells and colleagues described as "noticeable differences" in the washout region, confirming a difference between the healthy control subjects and cardiac amyloidosis patients.
Based on the TAC and images, the optimum times to calculate SUV of florbetapir were in the first five minutes of the imaging procedure, and again between 15 and 20 minutes. The SUV calculations between two time points appeared to be a feasible clinical method for use in discriminating between healthy individuals and patients with cardiac amyloidosis.
By calculating the percentage change, ratios, and slope of SUVs at the two time points, the researchers found a statistically accurate model in which the average SUV ratio for control subjects was 5.49, compared with an average SUV ratio of 2.19 for the cardiac amyloidosis patients.
"When we look at the initial five patients [in the study] and two control subjects, we were able to determine a significant cutoff ratio of 3.84," Wells said. "This model was then applied to [individuals] whom we imaged without knowledge of their cardiac amyloid status. This model was able to correctly classify the three cardiac amyloid patients with SUV ratios below 3.84, as well as our control patients with an SUV ratio of 5.92."
In addition, cardiac amyloidosis subjects showed a change in myocardial mean SUV of less than 55% on images from zero- to five-minute acquisitions and 15- to 20-minute acquisitions, compared with a mean SUV change of more than 70% in the control subjects.
Wells and colleagues concluded that florbetapir is a "clinically feasible model for obtaining cardiac amyloid utilizing a short imaging time protocol," with the two-time-point SUV analysis allowing the identification of cardiac amyloid based on an SUV ratio of less than 3.84.
Even with the small study sample, the results indicate "the potential for a clinical method of diagnosis in the quantification of myocardial amyloid load, which warrants further investigation," Wells added.
Study disclosures
The study was supported by the Aslan Foundation, a Pfizer Amyloidosis Fellowship, and the Physician's Medical Education and Research Foundation.