A study in the March issue of the Journal of Nuclear Medicine provides more evidence that the radiotracer florbetapir can be a "robust imaging tool." It appears well-suited as a biomarker to detect characteristics of Alzheimer's disease and someday could possibly guide treatment of individuals with the disease.
Currently, the only reliable way to make a definitive diagnosis of Alzheimer's is through postmortem examination. Imaging techniques have shown success in detecting early signs of Alzheimer's pathology, but these frequently have drawbacks: For example, the PET agent carbon-11-labeled Pittsburgh Compound B (PiB) has a half-life of just 20 minutes, which could limit its use in community healthcare centers, according to co-author Abhinay Joshi, from Avid Radiopharmaceuticals, and colleagues (JNM, March 2012, Vol. 53:3, pp. 378-384).
Joshi led a team of researchers from several institutions -- including the University of Pennsylvania; Molecular NeuroImaging in New Haven, CT; and Yale University -- who tested whether the radiopharmaceutical florbetapir was effective in PET imaging of beta amyloid in the brain. Avid, a subsidiary of pharmaceutical firm Eli Lilly, developed the radiotracer, which Eli Lilly is moving to market under the trade name Amyvid.
Florbetapir is an amyloid PET ligand with rapid brain uptake and washout from gray tissues not containing amyloid. Joshi and colleagues also noted that the radiotracer has a "short imaging time, good separation between the amyloid retention and background signal," and extended stability, which allows for flexible imaging time.
Two protocols
The researchers collected data to study two aspects of florbetapir imaging performance. One protocol was to gauge the appropriate dose for optimal imaging, while the second protocol (test-retest) was to evaluate and confirm the reproducibility of florbetapir with PET in younger healthy subjects and patients with Alzheimer's.
In both protocols, volunteers were recruited from six different sites. Volunteers within the Alzheimer's disease group were 50 years of age or older, while healthy control subjects with no history of cognitive impairment were between the ages of 35 and 55.
In the first protocol, Joshi and colleagues randomized study participants based on whether they received a low dose of florbetapir (111 MBq, or 3 mCi) or a standard dose (370 MBq, or 10 mCi).
Of the 20 participants in the study, nine (five with Alzheimer's and four control subjects) received a single injection of 111 MBq, while 11 (four with Alzheimer's and seven control subjects) received a single dose of 370 MBq. All 20 individuals underwent PET immediately after receiving the radiotracer, and images were captured for 90 minutes.
In the second protocol, 10 Alzheimer's patients and 10 control subjects participated in the test-retest study. Each participant was imaged on two separate days not more than four weeks apart, receiving a single dose of 370 MBq of florbetapir, followed 50 minutes later by a 20-minute PET scan.
Three board-certified nuclear medicine physicians who were unaware of the subjects' clinical and demographic information visually evaluated all the PET images.
Dose evaluation
The group found that standardized uptake values (SUVs) and SUV ratios for target cortical brain regions were greater for Alzheimer's subjects than among healthy controls at both the low and standard levels of florbetapir dosage.
In individuals with Alzheimer's, the mean cortical average SUV ratio at 50 to 60 minutes after radiotracer injection was 1.78 ± 0.22 for the 111 MBq dose and 1.66 ± 0.29 for the 370 MBq dose. Among the control subjects, the mean cortical average SUV ratio was 0.97 ± 0.05 for the 111 MBq dose and 0.99 ± 0.09 for the 370 MBq dose.
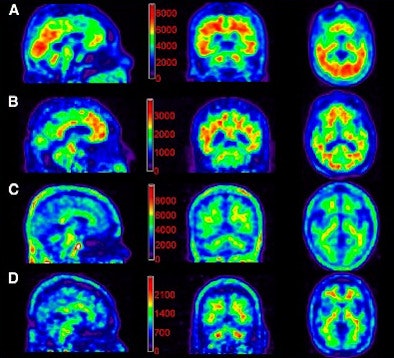 |
| Florbetapir images for 111-MBq and 370-MBq dose groups acquired in a 10-minute PET scan 50 minutes after injection. Counts per pixel are shown on color scale for purposes of this figure, but were not available to readers. Image A is a 370-MBq dose in an Alzheimer's subject. The average image quality rating was 4. Image B shows a 111-MBq dose in an Alzheimer's subject, with an average image quality rating of 2. Image C shows a 370-MBq dose in a control subject. The average image quality rating was 4. Image D is a 111-MBq dose in a control subject, with an average image quality rating of 2. Images courtesy of the Journal of Nuclear Medicine. |
The authors did note a difference in visual quality between the 370-MBq dose and 111-MBq dose, with the latter showing slightly lower image quality. However, "no statistical significance was achieved in this relatively small sample of subjects," they wrote.
Readers interpreting the PET florbetapir scans were able to identify all Alzheimer's subjects as beta-amyloid positive and all control subjects as beta-amyloid negative.
Test-retest analysis
In the test-retest portion of the study, SUV ratios for the cortical average relative to cerebellum of the test and retest scans were 1.42 ± 0.25 and 1.41 ± 0.27 for Alzheimer's subjects, compared with 1.00 ± 0.06 and 1.01 ± 0.06 for control subjects. Similar results were obtained for individual cortical regions.
The authors also noted a "high level of agreement" between the visual determination of beta-amyloid positive or beta-amyloid negative results for the test-retest images. "Each reader assigned the same classification to the test and retest images on 19 of 20 image pairs (95%)," they wrote.
There was also a less than 3% difference between test and retest images for the cortical average SUV ratio as tested at 50- to 60-minute and 50- to 70-minute postinjection acquisition times.
"Thus, a 10-minute florbetapir scan 50 minutes after injection appears sufficient for evaluating the degree of amyloid pathology," the authors concluded.
Joshi and colleagues cited two limitations of their research. One limitation of the dose comparison part of the study is that the research was performed in two different cohorts of subjects. They noted that in such a small sample "interpretation can be confounded by a few anomalous subjects."
The second limitation of the study, they added, is that the use of young healthy control subjects rather than older healthy individuals or people with mild cognitive impairment "may have reduced sensitivity for detecting some differences between doses and variation in test-retest performance."
In conclusion, florbetapir showed a "wide effective dose range and high test-retest reliability for both visual assessment of ligand retention and [SUV ratio] values," the authors wrote. "Both the dose-ranging study and the test-retest study indicate that florbetapir F-18 is a robust imaging tool well-suited for use as a biomarker for [Alzheimer's] pathology in standard community PET facilities."
|
Study disclosures Lead author Joshi and several co-authors are employees of Avid Radiopharmaceuticals, which also funded the study. |