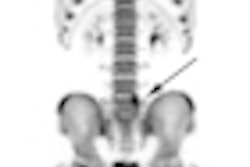
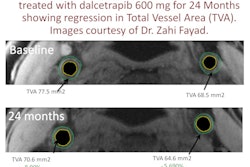
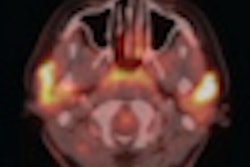
PET developer Naviscan has received the CE Mark for both its high-resolution positron emission mammography (PEM) scanner and PET-guided biopsy accessory.
The approval enables the company to market the system in Europe, where it has set up distribution in 13 countries, Naviscan said. The firm has already received the first two orders for the system in Germany and Switzerland.Naviscan's PEM gets CE Mark
Latest in Nuclear Medicine
Journal of Nuclear Medicine names new editor in chief
February 11, 2026
Niowave inks Ac-225 supply deal with Novartis
February 11, 2026
New study seeks to tailor RLT to individual patients
January 26, 2026