In acute stroke imaging, there's a big difference between actual brain physiology and the simplistic representations thereof produced by CT and other imaging tools. Researchers are doing what they can to close this technical gap, hone their imaging techniques, and speed treatment with the aim of reducing morbidity and mortality from acute stroke -- currently the second leading cause of death worldwide.
Considering the mismatch between the brain imaging signal and brain anatomy, imaging research is focused on creating more realistic depictions of brain tissue at risk. Investigators must also validate the protocols in current use -- by comparing them to each other, to no treatment at all, and, finally, to patient outcomes. Along the way, they will need to show that by imaging a small subset of candidates for therapy, outcomes are improved for all patients.
Our two-part series on acute stroke imaging, based on discussions of controversies in stroke imaging presented at the 2007 RSNA meeting in Chicago, looks at the current issues in scanning, defining, and treating potentially salvageable brain tissue.
Soon after arterial occlusion occurs there is a graded reduction in cerebral blood flow from the densely ischemic infarct core to normally perfused surrounding tissue, explained Dr. William Powers, professor and chair of neurology at the University of North Carolina at Chapel Hill.
"When we talk about imaging acute stroke to try to find salvageable tissue, we basically fall back on a ... very simplistic model," he said in his RSNA presentation. Unfortunately, he said, this model does not accurately depict brain physiology, and getting an imaging modality to do so is an extraordinarily difficult technical challenge.
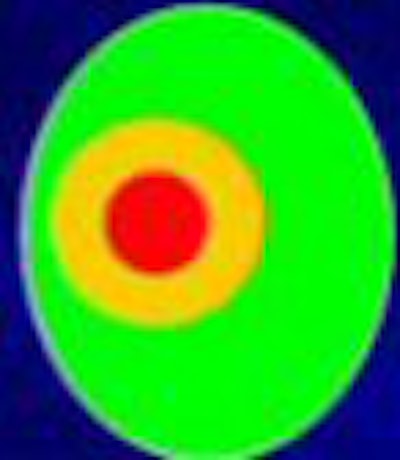
 |
| The classic three-component tissue model of ischemic stroke model defines the central infarct core in which cells die quickly. In the penumbra region, where lack of oxygen and glucose produces rapid depletion of energy stores, cell death can be prevented by expeditious treatment. Beyond the penumbra is benign oligemia, a mildly ischemic region at low risk of tissue death, surrounded by normal brain parenchyma. All images courtesy of Dr. William Powers. |
"We assume that in this dense center there are cells that effectively die right away or very quickly," Powers said. "In the (penumbra) area around it are cells that aren't dead yet but will go on to die in some definable, relatively short time period. Beyond that is the (benign oligemia) brain that isn't particularly at risk at all. The idea is that you've got this zone of tissue that is not dead yet, but if it is untreated it will become so, and if it's treated it will survive and not die."
In fact, penumbra is a "terrible term," Powers said. Better to call it a zone of preventable infarction: tissue that will die if untreated and live if treated.
Moreover, this zone of preventable infarction is not static but changeable, as well as time- and treatment-dependent, Powers said (Cellular and Molecular Neurobiology, November 2006, Vol. 26:7-8, pp. 1055-1081; Journal of Cerebral Blood Flow & Metabolism, August 2006, Vol. 26:8, pp. 1018-1030).
Tissue at risk
Reversible ischemia is another misleading term in the stroke lexicon "because all ischemia is reversible," Powers said. "You can always re-establish blood flow to tissue, but it doesn't do any good if the tissue is stone-cold dead when you do it."
"Ischemic penumbra" is more accurate, but the phrase must be used cautiously, he said. European researchers began using penumbra in the early 1980s to describe the region of infarcted brain tissue that looked like a ring around an eclipse. The ischemic penumbra represented tissue with cerebral blood flow low enough for the neurons to stop firing but still above the threshold of energy failure and ion pump failure, Powers said, referencing Astrup et al (Stroke, November-December 1981, Vol. 12:6, pp. 723-725).
The problem arises when one tries to apply the concept of ischemic penumbra to imaging, "because you can define the ischemic penumbra however you want to, and all the definitions are physiologic -- they're not dependent on the ability to salvage tissue," Powers said, noting that early authors were careful to distinguish penumbra and reversibility by specifying that "tissue that is not penumbra can be salvaged by reperfusion."
The ideal technique will produce signals that correspond to the three types of tissue involved: "One signal that tells us what's already dead, one signal that tells us what is potentially salvageable with appropriate treatment, and one signal that tells us what isn't at risk at all," Powers said.
Assuming such a technique exists, how could a study determine whether the modality permits such distinctions between tissues? Because the differentiation of preventable infarction versus dead tissue depends on the specific treatment, researchers will need a treated group and an untreated group of patients to test it -- and for the test to be valid, the treatment will also have to be effective, he said.
Today, despite almost 20 years of neuroprotective therapy, effective treatment still means reperfusion therapy three hours or less after symptom onset, Powers said.
The right study
Proving the effectiveness of an imaging protocol will require at least three sets of patient images: one at admission to find preventable infarction, a second set showing that the reperfusion attempt was successful -- "or shows the tissue that was reperfused versus tissue that wasn't within a relatively short time period" -- and finally late image data showing the tissue that eventually died due to lack of oxygen despite the reperfusion therapy. This kind of tissue is dead from the start, Powers said, so reperfusion is successful but unhelpful.
PET, though certainly not the only game in town, might be able to provide such proof, Powers said in reference to a study of reperfusion performed in baboons. Giffard and colleagues concluded that apart from duration of occlusion, "the fate of the at-risk tissue is predicated by the initial severity of the ischemia, as well as by early secondary events such as partial spontaneous reperfusion" (Journal of Cerebral Blood Flow & Metabolism, May 2004, Vol. 24:5, pp. 495-508).
Studies using PET CBF-CMRO2 have shown that blood flow and oxygen can be combined to identify the narrow zone of preventable infarction, as opposed to dead tissue or tissue that will survive untreated, Powers said. However, additional human reperfusion studies are needed to show that the technique works, and the barriers are formidable, including a cyclotron that operates 24/7, arterial catheterization, and complicated postprocessing. More labs need to be set up, and more researchers trained to work with the agent, he said.
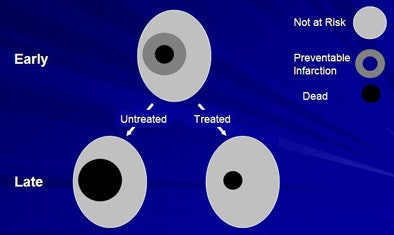
 |
| Imaging with PET-CBF-CMRO2 passes the criteria for accurate neuroimaging of preventable infarction, but current data are a mix of human and animal studies, and there are significant barriers to clinical implementation. |
Study design challenges also complicate the validation of imaging techniques, Powers said.
"The problem with the intermediate (penumbra) zone is that, basically, if you treat some of it, all of it will survive, but if you don't treat, most of it will survive anyway," he said. As a result, the 20% of tissue that died untreated can't be differentiated from the 80% that survived.
"Looking at preventable infarction with (CBF-11 CFMZ) PET fails, because you still end up having only 20% (of tissue) die untreated, and it's a signal that doesn't accurately distinguish tissue that will go on to survive untreated versus tissue that will go on to die treated," Powers said.
Unfortunately, he said, the same limitation applies to diffusion-perfusion mismatch imaging in MRI, despite its popularity and multiple arguments in its favor. What the diffusion-perfusion mismatch really means is that the status of tissue inside the region of reduced perfusion is indeterminate, Powers said.
"Some of it goes on to infarct and some of it doesn't, but that doesn't mean that any of it within that zone was actually salvageable," he said. "It may just mean that the way you're trying to identify it isn't any good."
Even if a turnkey cyclotron could be developed to yield 100% accuracy in defining salvageable tissue, proof of clinical utility would still be needed to prove that the test was effective, Powers said. The problem is that many neuroimaging protocols that claim to identify preventable infarction erroneously equate indeterminate outcome with preventable infarction, he said.
"The only way clinical utility can be proven is by demonstrating that patients who had that type of imaging applied to their tear actually do better -- and that is a very difficult thing to do," Powers said. Such a study could theoretically be designed, he added, but don't look for it anytime soon.
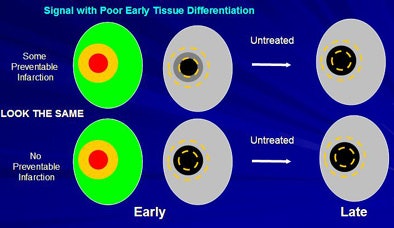 |
| Neuroimaging signals showing indeterminate outcome in untreated patients cannot establish the existence of preventable infarction; the signal may simply be incapable of accurately differentiating tissue types at early times. |
A convincing study would require two groups of acute stroke patients: one treated and one untreated -- neither of which received image-guided therapy -- to show that therapy without image guidance doesn't work. A third group of patients who receive image guidance to determine whether they will be treated will have to be incorporated. This last group must yield outcomes even better even than the group that was treated without image guidance, Powers said.
After all, he explained, if the imaging itself has an adverse effect on outcomes -- by increasing morbidity and mortality, complicating adverse events, or delaying treatment such that the benefit of treatment is reduced -- then it will be impossible to show that image-guided therapy is clinically useful.
Such a study would also require a lot of money and a very large cohort, Powers said. And a benefit from image-guided patient selection will be difficult to demonstrate, absent a high percentage of patients with preventable infarction who do very well after treatment.
Finally, if the adverse effects of therapy are nonexistent, then it doesn't matter if patients are selected correctly for treatment. One might as well treat everyone, he said.
A series that Powers' group performed in a large group of patients showed no benefit, he said, so they designed a new study that performs PET in a subgroup of patients randomized to treatment or no treatment to try to show a benefit from imaging. Even so, he said, any adverse effects from the imaging modality itself cannot be accounted for with this study design.
Given all the limitations, what can imaging providers do now? To date, three reperfusion therapies have shown a benefit in outcomes, according to Powers: IV recombinant tissue plasminogen activator (rtPA) within three hours of symptom onset, ancrod (snake venom extract) within three hours of onset, and intra-arterial prourokinase (not approved for use in the U.S.) within six hours of onset for M1 and M2 occlusions.
The only proven treatment available for clinical use in acute stroke patients is IV-rtPA within three hours of onset -- and it requires a noncontrast CT scan.
"That's the kind of imaging we should be doing to guide our therapy in acute stroke," he said. "Anything else is based on unproven hypotheses; carries real costs, primarily in delay to therapy (and) in making decisions to treat people whom we may be hurting not helping; and therefore should only be performed ... as part of (Institutional Review Board)-approved clinical studies in which people sign informed consent and realize they're being subjected to experimentation and not clinical standards."
By Eric Barnes
AuntMinnie.com staff writer
February 21, 2008
Related Reading
SWI MR reveals microarchitecture of neurovascular diseases, February 1, 2008
CT-stroke scale fails to identify salvageable brain injury after stroke, July 4, 2007
Diffusion-weighted MRI: The cornerstone of neuroimaging-based stroke studies, June 14, 2007
MRI and CT findings comparable in stroke patients, March 9, 2007
Small stroke centers ill-equipped for range of presentations, April 25, 2007
Copyright © 2008 AuntMinnie.com