The medical imaging industry is gearing up for two days of hearings March 30-31 held by the U.S. Food and Drug Administration (FDA) regarding rising medical radiation dose.
Each day of the hearings will be divided into two sessions, according to the American College of Radiology (ACR) of Reston, VA. The first day of the hearings will focus on equipment features that imaging vendors could incorporate into their products to reduce patient exposure to ionizing radiation during CT and fluoroscopy procedures, with CT discussed in the morning and fluoroscopy in the afternoon.
On the second day, the morning discussion will focus on steps that manufacturers can take to improve the training of personnel who use imaging equipment, while the afternoon will focus on steps to improve quality assurance at imaging facilities using CT and fluoroscopy, the ACR said.
Among the speakers representing radiology will be Dr. E. Stephen Amis, co-chair of the ACR/RSNA Joint Task Force on Adult Radiation, and Richard Morin, Ph.D., chair of the ACR Safety Committee. Amis and Morin will make scientific presentations on the second day of the meeting, and will serve on the primary roundtable during discussion, the ACR said.
Several speakers will also discuss the Image Gently campaign, which has raised awareness of pediatric radiation dose since its launch in January 2008. The speakers will include:
- Dr. Marilyn Goske, chair of the Alliance for Radiation Safety in Pediatric Imaging
- Dr. Marta Hernanz-Schulman, who is leading an upcoming Image Gently summit on fluoroscopy dose
- Dr. Donald Frush, chair of the ACR Pediatric Radiology Commission
- Keith Strauss, member of the ACR Subcommittee on Radiation Exposure
- Greg Morrison, chief operating officer of the American Society of Radiologic Technologists (ASRT) of Albuquerque, NM
Meanwhile, the New York Times on March 28 ran an article claiming that the FDA had ignored warnings by agency scientists who were reviewing an application for virtual colonoscopy software regarding the dangers of radiation dose from the technique. A gastroenterologist who worked under contract with the FDA in reviewing the application claims that his contract was not renewed due to his opposition to the application. He plans to tell his story during the committee hearings, the article said.
Related Reading
Medical groups tell Congress more radiation rules are needed, March 1, 2010
Congress surprised at lack of medical radiation oversight, February 26, 2010
MITA unveils radiation dose warning system for CT, February 25, 2010
FDA launches initiative to rein in medical radiation, February 9, 2010
ASTRO commits to patient protection plan, February 4, 2010
Copyright © 2010 AuntMinnie.com







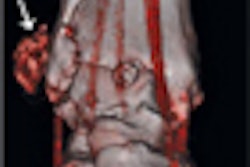


![Images show the pectoralis muscles of a healthy male individual who never smoked (age, 66 years; height, 178 cm; body mass index [BMI, calculated as weight in kilograms divided by height in meters squared], 28.4; number of cigarette pack-years, 0; forced expiratory volume in 1 second [FEV1], 97.6% predicted; FEV1: forced vital capacity [FVC] ratio, 0.71; pectoralis muscle area [PMA], 59.4 cm2; pectoralis muscle volume [PMV], 764 cm3) and a male individual with a smoking history and chronic obstructive pulmonary disorder (COPD) (age, 66 years; height, 178 cm; BMI, 27.5; number of cigarette pack-years, 43.2, FEV1, 48% predicted; FEV1:FVC, 0.56; PMA, 35 cm2; PMV, 480.8 cm3) from the Canadian Cohort Obstructive Lung Disease (i.e., CanCOLD) study. The CT image is shown in the axial plane. The PMV is automatically extracted using the developed deep learning model and overlayed onto the lungs for visual clarity.](https://img.auntminnie.com/mindful/smg/workspaces/default/uploads/2026/03/genkin.25LqljVF0y.jpg?auto=format%2Ccompress&crop=focalpoint&fit=crop&h=112&q=70&w=112)