The days of planning liver interventions on the strength of a few CT or MR reconstructions may be numbered. Researchers from Germany and beyond are honing a set of advanced planning tools that not only provide high-resolution color-coded 3D visualization of the liver parenchyma and vasculature, but offer quantitative views of the intrahepatic vessel branching system and the risks of cutting at a given point in the organ.
Applied to tumor resection or living donor transplantation planning, the software helps surgeons optimize the shape of the cutting plane and decide which vessels will need to be reconstructed to avoid congestion and the loss of liver function.
Early studies suggest that HepaVision (MeVis, Bremen, Germany), an investigational software package that runs on the Windows platform, can improve preoperative planning while minimizing the risks of intervention.
While HepaVision has been applied to more than 2,500 liver interventions to date, future iterations will offer greatly expanded functionality based on research that is under way, said Heinz-Otto Peitgen, Ph.D., director of the MeVis Research Center for Medical Image Computing in Bremen, Germany, a nonprofit institute at the University of Bremen.
Peitgen, a mathematician who founded the MeVis center, discussed current research in advanced liver imaging applications at the 2007 Computer Assisted Radiology and Surgery (CARS) meeting in Berlin.
"The application of fractal mathematics has grown very impressively in recent years," particularly with regard to the modeling of liver vasculature, Peitgen said. The result, computer-assisted 3D surgery planning, has been shown to improve preoperative assessment of functional resectability in liver regions at risk for devascularization or impaired drainage, he said.
Still, more research will be needed to address the myriad problems that are "more difficult than we imagined," Peitgen said. Success will depend on the active participation of researchers from many scientific fields and as many countries, he said.
A key problem in living donor liver transplantation, for example, is deciding the best place to cut and where vessels must be reconstructed. The location of the middle hepatic vein determines where the liver will be divided between the donor and recipient. Solving what Peitgen calls the "middle hepatic vein (MHV) dilemma" is crucial for success.
"You want to split a donor's liver so that those parts in the (donor's) remnant and the part for the recipient remain fully functional, splitting them in such a way that the vascular structures, such as the bile ducts, the hepatic artery, the hepatic vein, and the portal artery, remain functional," Peitgen said.
Cutting to the right of the MHV means dissecting some of the veins feeding into it, he said. The result is areas of liver dysfunction -- bad news for both recipient and donor. Surgeons in different countries have developed their own standard ways of dealing with the problem, Peitgen said. But advanced planning software can do a better job by individualizing the planning based on the patient's CT data.
HepaVision segments the vessels and analyzes the vasculature, displaying the areas that would be damaged if the dissected vessels were not reconstructed at surgery, Peitgen said. Another display provides a 3D representation of what the surgeon will encounter during the procedure.
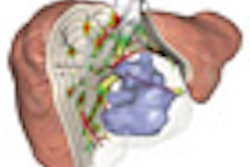
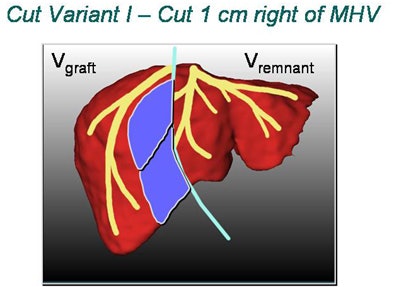 |
| In living donor liver transplantation, optimizing the location of the split between the donor's liver remnant and the recipient's graft can have a major impact on liver function for both postsurgery. Software shows areas at risk in various alternatives, such as cutting 1 cm to the right of the middle hepatic artery (above). 3D images below show varying degrees of risk for this choice in different regions of the liver vasculature. All images copyright Heinz-Otto Peitgen. |
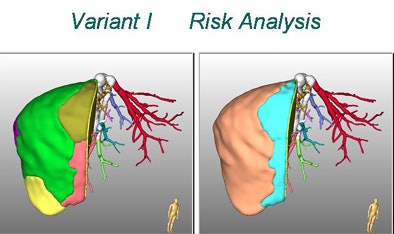 |
In a study published earlier this year, Peitgen along with researchers from Bremen and Germany's University of Essen called the planning software a "new way to resolve the dilemma of the middle hepatic vein" in adult living donor liver transplantation (ALDLT) (World Journal of Surgery, January 2007, Vol. 31:1, pp. 175-185).
"Computer-assisted planning allows for the 3D reconstruction of the vascular and biliary anatomy, automatic calculation of the total and territorial liver volumes, and risk analysis of hepatic vein dominance relationships," wrote Peitgen and colleagues from Bremen and Essen. "This data acquisition supports preoperative evaluation and provides a high degree of safety for donors and improved outcomes for recipients."
The study found substantial anatomic variation in its cohort. Only 29% of 75 potential donors had vascular and biliary anatomy consistent with classically described "normal" patterns, while 71% had anatomic variations, the researchers explained. Based on the automated preplanning, just under half of the candidates underwent the procedure.
Thirty-nine (52%) donors underwent ALDLT hepatectomy. The right hepatic vein was dominant in 64 cases, representing 48 ± 6% of the total liver volume (TLV). The middle hepatic vein was dominant in 11 cases, making up 40 ± 8% of the TLV. The left hepatic vein was never dominant. The volume contribution of the middle hepatic MHV was 114-782 mL for the right and 87-419 mL for the left hemiliver.
About 90% of liver transplantations in the U.S. and Europe are from cadavers, but in many parts of Asia where ethical reasons impede cadaveric transplantation, living donor transplantation is the only reliable way to do the procedure, Peitgen said.
Another paper, published earlier this month, discusses the value of advanced liver intervention planning. Automated preplanning improves preoperative assessment of functional resectability in areas at risk for either devascularization or impaired drainage, wrote Peitgen et al from Bremen and Essen.
"In selected cases, this information may have a considerable influence on operative planning, especially with regard to the extent of resection or the need for vascular reconstruction," they wrote in the abstract. "Due to the great anatomical variability of the intrahepatic branching patterns of the right liver lobe, this seems to be particularly important in extended left hepatectomies or in repeat hepatectomy when intrahepatic vascular anatomy may be altered. The development of navigation techniques to ensure the accurate application of the preoperative planned resection line is under investigation but not available yet" (Zeitschrift für Gastroenterologie, September 4, 2007).
In addition to transplantation planning, research is proceeding on other fronts as well. The MeVis team hopes to incorporate this knowledge into future versions of the software.
Oncology intervention planning
A recent unpublished study applies preoperative planning to oncology, specifically resection planning and risk assessment, Peitgen said. CT data is being used to predict the parenchyma at risk for a given tumor in an individual patient.
Automated tumor resection planning is complicated by limitations in imaging technology, which cannot precisely define the tumor boundaries, he said.
"We will never be able to say exactly with imaging technology where the lesion starts or ends, so the surgeon ... will take a safety margin some distance around the lesion," Peitgen said. "Suppose we take a 0.5-cm safety margin. Then we will cut into the local vascular structures, and that will resolve into a supply or drainage problem for the liver -- what I like to call 'parenchyma at risk.' And obviously if you take a larger margin you destroy more of the function of the liver. How is that risk to be calculated?"
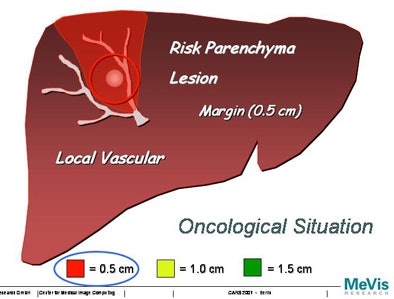 |
| Increasing the margin of safety around a tumor can greatly increase the areas of potential liver dysfunction. |
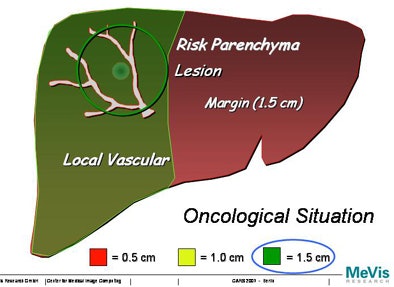 |
Swiss researchers are using a corrosion cast of liver vasculature taken from high-resolution CT data to predict risk for different safety margins around the visible tumor, Peitgen explained.
For the study, the researchers placed tumors randomly around the corrosion cast, then predicted the area of the liver that would be affected. The risk distribution graph is a function of the safety margin and the position of the lesion, Peitgen said, demonstrating how the new functionality changes the view of the liver as different surgical margins are chosen and different vessels are affected.
"When the lesion is in the (periphery) of the liver, nothing really surprising is happening," he said. "But when the lesion is closer to the center of the liver, all of a sudden the entire right or left liver becomes dysfunctional. Sometimes very minute changes in the location of the lesion have a dramatic impact in terms of functional failure."
The larger the surgical margin, the larger the portion of the vasculature that is rendered dysfunctional. And vessels are not the same. As one would expect, risk curves for the portal vein and hepatic vein are quite different, Peitgen said.
 |
| Top, a tumor placed in a corrosion cast of CT liver data is used to estimate the potential loss of function as a function of tumor location. Below, the extent of liver parenchyma at risk from tumor resection is shown as a nearly stepwise function of the size of tumor position and the safety margin surrounding the radiologically visible tumor. Bottom, the combined risks to both the hepatic and portal veins from tumor resection with a 1-cm margin are depicted in a 3D representation of the liver. |
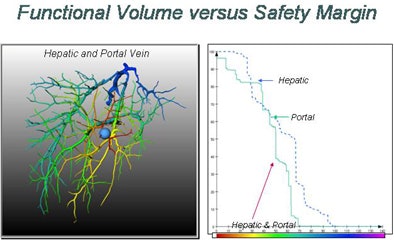 |
 |
In randomly placed liver lesions, remaining liver function and volumes versus the radius of the tumor margin yielded risk curves that were nearly stepwise in form, he said. "Risk is a nontrivial, nonlinear stepwise function of the values of the margin"; the curves offer "much more context than you would intuitively guess," all based on the patient's own images.
"We begin to understand that risk is highly nonintuitive, and that the distribution is highly individual and nontrivial in terms of position of the lesion as a function of the radius of the margin," he said. It is critical information for the surgeon to have beforehand.
Intraoperative adjustments
Anatomy is never static, and presurgical planning can't possibly cover everything that will be uncovered during the procedure, Peitgen said. So another area of research is examining ways to preplan CT images intraoperatively.
Say you're in the middle of an operation and you discover a new lesion, Peitgen said. "What are you going to do? Stop the surgery and go back to some computer lab and maybe work for another hour or two and do a new preoperative planning ... that lists the additional lesion?" he said. "Of course not."
The researchers have used an animal model, specifically, a pig undergoing laparoscopic surgery of the liver, to demonstrate updated preplanning images. The method uses magnetically tracked ultrasound to determine the diameter of the newly discovered lesion, recording the diameter of the lesion on a touch screen.
"That diameter and position are then pulled over into preop setup, and the (software) then runs a new risk analysis based on the new finding in addition to what we had before," Peitgen said. "Then there is a transfer of data into the intraoperative setup of the guided ultrasound, and the surgery can continue with that new information."
Regeneration modeling
"We know that the liver has the capability to regenerate," Peitgen said. The question for researchers is how to demonstrate how it happens. One approach is macroscopic, wherein tracking liver donors and recipients could "lead to models of regeneration, which then can tell us about risks of regeneration," he explained. "For that we need all sorts of studies including volumetric measurements and modeling" to explain how regeneration happens and what parts of the liver regenerate.
The formation of collaterals has been a mystery in the transplantation field for some time, Peitgen said, adding that his group was surprised to see cases of dissected, dysfunctional vessels postsurgery that formed new collaterals in the course of a year.
The preoperative and follow-up CT data shows regeneration of both liver parenchyma and collateral vessels, Peitgen said. The data are being fed into a database that will eventually be able to predict both regeneration over time and parenchyma at risk.
Once regeneration is better understood, the MHV dilemma may end up being easier to solve, Peitgen said. Still, understanding regeneration macroscopically won't be enough to understand or predict it reliably, he noted.
"Part of the study we have to do is going to the microscopic level, to understand how (regeneration) happens, how it is prevented, and how it becomes really multifaceted on the microscopic level," Peitgen said. "That's another beautiful challenge of understanding liver surgery."
Tumor ablation
Another daunting challenge is liver tumor ablation, an increasingly common method of liver intervention that spares some of the risks and cost of open surgery, while creating new problems of its own, Peitgen said.
"One of the biggest problems besides the proper needle placement is understanding whether or not a particular lesion can be ablated without harming the patient or his liver function," he said. "If I cannot, then why not? Maybe I can if I understand the problem."
As interventionalists are well aware, the problem in many cases is a large vessel adjacent to the tumor, with blood flow that cools the lesion so that it cannot be destroyed by the heat of the probe. The ability to precisely determine whether a tumor can be ablated, based on the individual patient's statistics, is a multidisciplinary challenge to be reckoned with, Peitgen said.
What is needed is an "honest" biophysical simulation of the ablation procedure based on physical instrumentation and individual patient information.
A truly accurate biophysical simulation of the ablation procedure will have to account for the spatial distribution of tissue temperatures and the impact of the ablation process on tissues. Tissue parameters depend on a nonlinear fashion on temperature, degree of coagulation, and degree of dehydration resulting from the process, and tissue changes its properties during the course of ablation, he said.
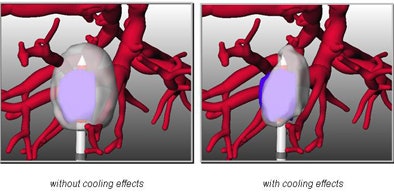 |
| Multidisciplinary research into the cooling effects of vessels adjacent to liver lesions and the effects of ablation on the thermal properties of tissue will be used to determine which tumors can be ablated, with the aim of optimizing procedure planning. |
"The answer will depend on widespread international cooperation of many fields, because biophysical simulation of ablation procedures involves the understanding of electromagnetic energy that is actually going into that patient's liver -- and that is not on the forms and manuals that you get with the device," Peitgen said.
"We could dream of creating a numerical optimizer that in a given clinical situation tells us how we should place the needle safely, automatically, by mathematical principals of optimization. This is all doable," he said.
Building the knowledge base
Research in all these areas is off to a good start, but more international cooperation will be needed, Peitgen said.
MeVis has more than 85 surgeons in its network, most in Germany but a few internationally, he said. Centers that aren't equipped with HepaVision planning software send their CT data via the Internet for analysis, and the data are added to the MeVis research database.
"We have started a database of risk calculations for preoperative planning," Peitgen said. "Via Internet we receive CT scans, we then perform risk calculations and send them back via Internet to the participating surgeons."
Over the past four years more than 2,500 liver surgeries, including 1,200 living donor transplantations, 900 complex oncological cases, and 200 other interventions have been performed using the software, he said. Regeneration has been tracked in more than 200 patients so far.
Of course, the need for advanced planning isn't limited to liver intervention. "The same kinds of paradigms and considerations soon will begin, and already have begun, for brain surgery, lung surgery, pancreatic surgery, and so on," Peitgen said.
By Eric Barnes
AuntMinnie.com staff writer
September 18, 2007
Related Reading
MDCT's 'virtual Whipple' offers practice run before complex surgery, August 16, 2007
CARS news: Automated device tracking beats conventional CT/US guidance, June 28, 2007
Part III: Image processing has room to grow, October 23, 2006
Part II: Medical image processing has room to grow, September 4, 2006
Part I: Medical image processing has room to grow, July 4, 2006
Copyright © 2007 AuntMinnie.com