
A California start-up firm called Photonify Technologies is gearing up for the 2003 market launch of an optical imaging system that could someday provide an adjunct to conventional mammography imaging. The company is led by a former ADAC Laboratories executive who believes the technology could provide functional information to help breast imagers differentiate malignant from benign tissue.
Photonify was founded in 1999 with the goal of commercializing research in optical diffusion imaging and spectroscopy (ODIS), a technology that characterizes tissue by measuring the scattering and absorption of photons. The Fremont company has developed a device that beams near-infrared light into tissue, then measures the light-scattering and absorption properties of that tissue as a means of assessing its hemoglobin and oxygen saturation levels.
Hemoglobin and oxygen saturation are critical indicators of tissue health, according to Mohamed Elmandjra, president of Photonify and a former ADAC executive. Elmandjra joined the firm in December 2001, a year after ADAC’s acquisition by Philips Medical Systems of Andover, MA.
Devices like pulse oximeters are currently used to measure oxygenation, but are of no use for tissue characterization because they provide only global measurements of oxygenation throughout the body, Elmandjra said. Photonify’s device differs in its ability to measure hemoglobin and oxygen saturation at a local level. The system can produce either a numerical rating of tissue oxygenation, or a color-coded image map that depicts oxygenation in the region of interest being examined.
The unit consists of a small transducer that both transmits near-infrared light into tissue and detects its diffraction and absorption. The system can be operated from a laptop computer with special software installed, or as part of an integrated device -- similar in size to a laptop computer-- that Photonify has developed.
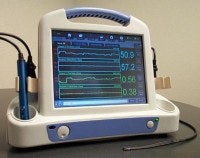 |
| Photonify’s ODIS instrument showing main unit and transducer. System is shown in monitoring mode, allowing real-time tracking of hemoglobin and oxygen saturation changes. Photo courtesy of Photonify. |
During operation, the probe is moved over the patient’s skin, with the system recording oxygen saturation levels and hemoglobin counts. If an area of low oxygen saturation and high hemoglobin is encountered, it could indicate some kind of pathology such as nonviable tissue or a malignancy.
Photonify sees the compact size of its device as a major competitive advantage over other optical imaging systems under development at other companies. Some of these systems incorporate a built-in patient table and are stationary once installed, while the Photonify unit can be carried to the patient, Elmandjra said.
There are a number of potential applications for Photonify’s measurements, with the three main uses being plastic surgery, peripheral vascular disease, and breast imaging. Plastic surgeons could find the system’s oxygenation measurements to be a useful indicator of whether tissue or skin that’s transplanted to a new location will survive.
Meanwhile, physicians assessing patients complaining of extremity pain would find Photonify’s data to be a good way to assess whether the patient’s limbs have adequate oxygen circulation, and thus whether they are at risk of gangrene or ischemia. The device could also be used to monitor patients after angioplasty or other interventional procedures to track restenosis or other patency problems.
Breast imaging applications
For breast imaging, Photonify believes that its technology can differentiate malignant tissue due to the different oxygenation profiles that characterize malignant and benign cells. The angiogenesis process in tumors causes malignant tissue to have higher hemoglobin levels due to increased blood flow. At the same time, oxygen saturation is lower than in surrounding healthy tissue because the tumor is consuming more oxygen. Both phenomena are reflected in Photonify’s image maps and data, Elmandjra said.
"This is similar to what PET does in measuring metabolism," he said. "This is essentially functional imaging. You are imaging oxygen saturation, and the corollary of that is cellular function."
Clinical studies have already been conducted at Massachusetts General Hospital in Boston, under the guidance of Dr. Daniel Kopans. In a trial completed in September, 50 women with indeterminate masses on their mammograms were examined with the Photonify unit prior to biopsy. The system had a negative predictive value in the upper 90% range, Elmandjra said.
"We had a lot of true negatives," he said. "When we said it was negative, it was really negative. Our negative predictive value was almost perfect."
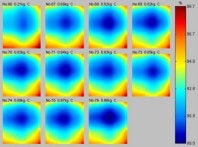  |
| Identification of malignancy in breast. Last seven frames on map at left show a decrease in local oxygen saturation, indicating hypoxia, while image at right shows a corresponding increase of hemoglobin concentration associated with vascularity. Diagnosis confirmed by biopsy: Focal mild ductal epithelial hyperplasia and focal sclerosis. Images courtesy of Photonify. |
Photonify sees the system being used as an adjunct to mammography, with its high negative predictive value helping to rule out women with suspicious masses who might otherwise be sent to biopsy. Elmandjra compared the system’s potential role to the way ultrasound is used for breast imaging, although the Photonify unit could prove even more useful due to its functional imaging capabilities.
Indeed, Photonify is encouraged by the prospect of merging the system with anatomical modalities to create hybrid functional/anatomic systems. The unit’s small size makes it easy to incorporate the technology into a larger anatomical scanner, such as a mammography or CT unit, Elmandjra said.
Photonify’s business plan calls for entering the plastic surgery and PVD markets first, as the company believes it can bring a product to market after going through the Food and Drug Administration’s 510(k) process. For breast imaging, a longer regulatory submission, such as premarket approval (PMA), may be required, which can be a much more lengthy process. Photonify is planning to officially introduce the system at the 2003 RSNA meeting, and is hoping to sell the product at less than $100,000 for an integrated system, Elmandjra said.
By Brian CaseyAuntMinnie.com staff writer
January 14, 2003
Related Reading
ADAC veteran Elmandjra to lead optical startup, December 17, 2001
Copyright © 2003 AuntMinnie.com



















