Ultrasound and microbubble contrast agents have proved to be a valuable team in many diagnostic applications. But these technologies may also ultimately combine to give a significant boost to the nascent field of gene therapy, according to research presented at the Advances in Contrast Ultrasound: ICUS Bubble Conference in Chicago.
Using ultrasound to target and destroy gene-laden microbubbles may help overcome many of the technical hurdles that have hampered clinical gene therapy, according to a Baylor University research team. In a study evaluating targeted gene therapy for human pancreatic islets transplanted into mice, this technique yielded improvements in revascularization and function of the human islets, as well as a significantly higher diabetes cure rate.
"It's a really promising way of targeting gene therapy to the organ that needs it, and not just to the whole body," said Dr. Paul Grayburn of Baylor University Medical Center in Dallas. He presented the group's findings at the Bubble Conference, held October 22 and 23 in an educational partnership with the International Contrast Ultrasound Society (ICUS).
Clinical gene therapy has long been plagued by difficulties in safely and effectively transporting genes to their intended destination. Genes injected into the bloodstream are destroyed immediately by enzymes, and even if they were able to survive the enzyme attacks, the genes still have to get across the endothelial barrier (the lining of blood cells) to reach their intended target, Grayburn said.
That problem can be solved, however, through the use of ultrasound and microbubbles (utilized in ultrasound contrast agents), Grayburn said. By incorporating genes into the shell of microbubbles (protecting them from the enzymes that would otherwise destroy them) and using high-mechanical index ultrasound to destroy the microbubbles in the target area, genes can be propelled through the endothelial barrier to their target destination.
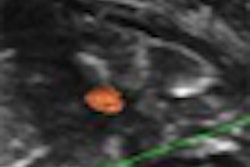
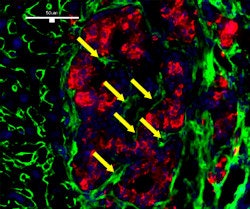 |
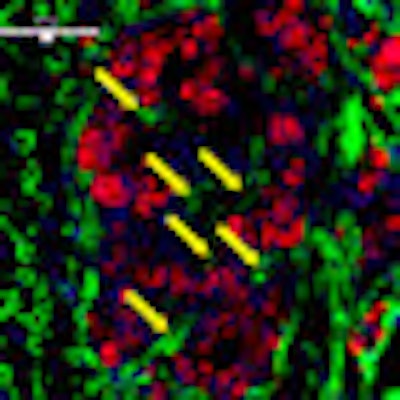
| Revascularization of graft islets (day 30). Red: human insulin; green: CD31; blue: DAPI; arrow: vessel in islets. Above image: hVEGF group. All images courtesy of Dr. Paul Grayburn. |
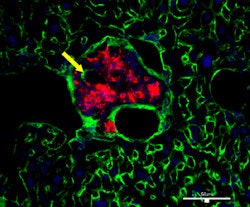 |
| GFP group. |
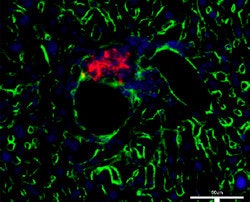 |
| Control group (no UTMD). |
The lack of intraislet microvasculature is a major cause of the loss of these pancreatic graft islets. While ex-vivo delivery of the human vascular endothelial growth factor (hVEGF) gene has been shown to improve graft function and survival after islet transplantation, these studies utilized unsafe viral vectors for gene expression and were, therefore, unsuitable for humans, according to Grayburn.
Ultrasound-targeted microbubble destruction offers a number of advantages in this application, including organ specificity and efficient gene delivery, Grayburn said. It also doesn't need a virus and is a simple and noninvasive technique.
The Baylor research team sought to examine the effect of hVEGF on human donor islets (deemed not to be of high enough quality for human use) that were transplanted via the portal vein into the liver of immunodeficient mice. This is a standard model that closely resembles clinical islet transportation in humans, Grayburn said.
The study cohort was divided into three groups; one group received microbubbles laden with hVEGF, while the second received microbubbles that included just a marker protein (green fluorescent protein [GFP]) to serve as a negative control. While these two groups received ultrasound-targeted microbubble destruction, a third control group received a standard mouse transplant technique without any gene therapy.
Eight of the 11 (72.7%) mice in the hVEGF group were cured of their diabetes after 30 days, compared with one of seven (14.3%) in the GFP group and one of eight (12.5%) in the group that did not receive gene therapy. The difference was statistically significant (p < 0.05).
"At four weeks, their blood sugar level was still below 200 [mg/dL], compared to only one cure in each of the other two groups," he said.
In other findings, serum human insulin and serum human C-peptide levels were significantly higher for the hVEGF group compared with the other two groups (p < 0.025). Revascularization was also superior in the hVEGF group.
"I believe the future of microbubbles as imaging agents is going to be in therapy, where we use them to deliver genes, drugs, fRNAs, peptides, and different types of compounds specifically where we want them to go," Grayburn said. "[For example,] imagine if you could put chemotherapy only in the cancer and not get any of those side effects [typically associated with chemotherapy treatment]. That's the kind of thing you can do with these microbubbles."
The Baylor researchers next would like to evaluate this technique in primates, with human testing, they hope, following down the road after approval from the U.S. Food and Drug Administration (FDA).
By Erik L. Ridley
AuntMinnie.com staff writer
October 29, 2009
Related Reading
Contrast ultrasound may spot early signs of atherosclerosis, October 20, 2009
Copyright © 2009 AuntMinnie.com