
Not many companies get a second chance at commercial success. But after nearly going out of business just a few years ago, gamma camera developer Dilon Technologies appears to have beaten the odds with the commercial introduction of a new dedicated scintimammography system.
The Newport News, VA, company is touting its Dilon 6800 gamma camera as small-field-of-view unit that can serve as an adjunct to mammography. Dilon hopes the procedure will be integrated into the diagnostic workup after a suspicious area has been identified on a mammogram, reducing patient stress about whether a lesion is benign or malignant and eliminating unnecessary biopsies.
The idea of using nuclear medicine for breast cancer applications has been an attractive one for clinicians since the 1990s. Imaging specialists began investigating the technology after conducting cardiac scans with 99mTc technetium sestamibi (Bristol-Myers Squibb Medical Imaging, N. Billerica, MA). They discovered areas of radiotracer uptake in the breast and realized that the radiopharmaceutical could be useful in breast imaging.
In 1999, the Food and Drug Administration cleared the use of 99mTc sestamibi for breast imaging under the trade name Miraluma, and mammographers began using it with standard whole-body gamma cameras. In that same year, Dilon debuted at the RSNA show with a work-in-progress dedicated breast gamma camera that it hoped would ride a new wave of interest in nuclear medicine breast imaging.
A false start
Initial hopes for scintimammography were high, due to the fact that it examines the functional, metabolic process of the tumor while x-ray mammography measures the structure and density of the tissue. There was one problem, however, in that whole-body gamma cameras adapted for breast imaging couldn’t accurately detect lesions smaller than 1 cm. Because of this, scintimammography didn’t pan out as the diagnostic complement to mammography that the clinical community had hoped for at that time, according to Nancy Morter, director of marketing and corporate communications for Dilon.
"Much like mammography, which began first with standard CR (systems) before dedicated units were developed, scintimammography couldn’t produce the desired results without dedicated instruments," Morter said. "But with a dedicated camera, radiologists can see lesions down to 3 mm, which allows them to detect earlier-stage cancer."
Dilon is calling its approach breast-specific gamma imaging (BSGI) in an effort to draw distinctions between its technology and scintimammography with whole-body units. A dedicated gamma camera allows better resolution and more views in comparison to a standard whole-body SPECT unit, according to Dilon.
Other companies also marketing dedicated breast imaging gamma cameras include Gamma Medica of Northridge, CA, which is selling a camera called LumaGEM 3200S, while IS2 Medical Systems of Ottawa, Ontario, is marketing its Breast Cancer Camera (BCC).
Since cancer cells absorb more technetium and absorb it faster than other cells, the 99mTc sestamibi images help radiologists determine whether a lesion is benign or malignant. Because scintimammography is not subject to changes in tissue that can occur in a woman’s body month to month or year to year, it’s an ideal adjunct technology to mammography, according to Dilon: Rather than having a biopsy to determine the presence or absence of disease, a woman can undergo a scintimammography procedure. And since scintimammography can be integrated into the existing mammography protocols, a woman can have results that same day.
  |
|
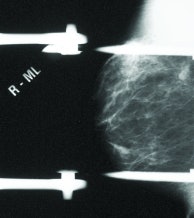
Patient is a 51-year-old female with prior left-breast lumpectomy of infiltrating ductal and DCIS. Mammogram (left) indicated nodular density in right breast, stable for three years. No abnormality detected, BI-RADS 2. Scintimammography with Dilon 6800 system (right) revealed area of high-density 6-mm focal uptake. Histopathology results indicate right breast intraductal and infiltrating ductal with DCIS carcinoma. Sentinel node was negative.
|
Dilon 6800 is configured much like a mammography unit, allowing the woman to sit upright with her breast lying on the detector and Dilon 6800’s gamma isolation shield on top holding it in place. The gamma shield protects the detector from background artifacts and reduces background scatter radiation.
The camera’s detector incorporates 3,000 3 x 3-mm pixelized scintillation crystals and 48 miniature photomultiplier tubes, and measures 6 x 8 inches with 0.4 inches of dead space. The unit’s detector and collimator can be positioned against the chest wall and rotated 360° as the breast is imaged. Once the radiopharmaceutical has been administered, the radiologist has two hours available to image; Dilon 6800 takes 10 minutes per view to image. The camera doesn’t require a dedicated room or staff, and can be moved from room to room if needed, much as an ultrasound device.
A bumpy road to market
Since its inception in 1996, privately held Dilon has collaborated on research and development with nuclear physicists at the Department of Energy’s Thomas Jefferson National Accelerator Facility, also in Newport News. The company now has a licensing agreement with the laboratory that allows it to use technology developed with Jefferson Laboratory in Dilon 6800.
In 1999, Dilon 6800 was cleared by the FDA for use in breast applications, and Dilon brought it to the RSNA show that year with high hopes for a commercial product launch. But the product's path to market hasn’t been easy.
In 2000, Dilon was negotiating for a large private offering with a New York investment firm when the high-tech stock market bubble burst and the deal fell through. Dilon’s staff worked through the year to keep the company financially viable, but then the September 11 terrorist attacks dealt the U.S. economy another blow, once again delaying Dilon’s efforts to launch the product.
The company secured private investment capital in 2003, and now has a sales and marketing effort in force. Dilon kicked off its marketing efforts in February at the National Consortium of Breast Centers (NCBC) show in Las Vegas, and will show the camera at the American College of Radiology’s National Conference on Breast Cancer this month in Orlando, FL, as well as this year’s RSNA show. The company plans to market the unit to both independent and hospital-based breast imaging centers. The technology already has a CPT code, and its reimbursement rate ranges from $400 to $600, according to Dilon.
"In a standard breast imaging protocol, a woman has a mammogram, and if she’s at risk for cancer, she’s referred for a biopsy," said Lon Slane, Dilon's CEO and one of its founders. "Our hope is that BSGI with Dilon 6800 can be integrated into the mammography protocol, so that clinicians can determine whether the suspicious area is benign or malignant and eliminate the need for biopsy at all."
By Kate Madden YeeAuntMinnie.com contributing writer
June 7, 2004
Related Reading
Dilon begins marketing breast gamma camera, April 23, 2004
Scintimammography augments traditional cancer detection methods, April 20, 2004
New camera may improve scintimammography, August 6, 2002
Dilon makes RSNA debut, December 2, 1999
Copyright © 2004 AuntMinnie.com





















