Case Particulars
A 36-year-old male presented with claudication of both the lower limbs and was detected to be hypertensive. There was no contributory history. General examination revealed a thin-built, averagely nourished individual with the pulse regular (76 beats per minute) associated with a pronounced radiofemoral delay, appreciated best during simultaneous arm and leg pulse palpation. His blood pressure recording at right upper limb was 170/90 mm Hg, at left upper limb 110/60 mm Hg, and both lower limbs 130/90 mm Hg.
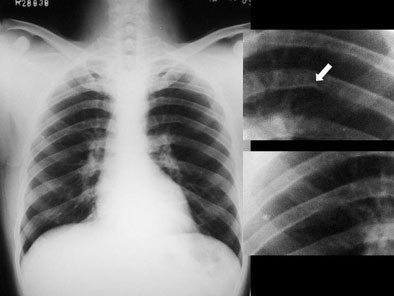
Systemic examination revealed evidence of a grade 2/6 ejection systolic murmur at the aortic area. Resting echocardiogram (ECG) revealed left ventricular hypertrophy and left atrial enlargement. Two-dimensional echo showed tricuspid aortic valve with no valvular regurgitation and coarctation of the aorta. The chest roentgenogram revealed a focal convexity to the descending aorta just distal to the aortic arch. Rib notching was present at both sides. [Figure 1]
All images courtesy of Surg Cdr I.K. Indrajit, Surg Cdr N. Mahesh, Surg Capt J. DSouza, and Col M.N. Sree Ram.
 |
| Figure 1. |
CT studies were performed using a Somatom Sensation 4 multidetector scanner (Siemens Medical Solutions, Erlangen, Germany) that was equipped with a flying spot adaptive-array matrix, Vistron pressure injector (Medrad, Indianola, PA, U.S.), and CARE Bolus software. CARE bolus facilitating an automated run was utilised in all cases with a threshold selected at 120 Hounsfield units (HU). Nonionic contrast media (100 mL of 300 mg I/mL) was injected through a 18-gauge antecubital Venflo catheter, using the pressure injector in a biphasic technique with initial 50 mL at a rate of 3.5 cc/sec, followed by remaining 50 mL at a rate of 2.0 cc/sec. A saline bolus chaser of 50 mL was employed to force the contrast through the tubing and the venous system to prolong the plateau phase of enhancement.
The scans were commenced with the patient positioned supine, from the pulmonary apex and extending inferiorly to the pubic symphysis, using a 4 x 2.5-mm combination of detectors. The scanning parameters were four detector rows, 120 kV, 130 mAs, 28-sec scan time, 0.5-sec rotation time, 3-mm slice collimation, and craniocaudal direction. The reconstruction parameters included 1.5-mm slice thickness and B30f soft kernel. 2D and 3D reformation was performed using maximum-intensity projection, shaded surface display, and volume-rendering algorithms on a workstation equipped with dedicated software (Leonardo, Siemens).
An analysis of the multidetector CT (MDCT) study and postprocessed images revealed the following findings:
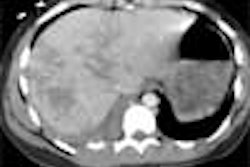
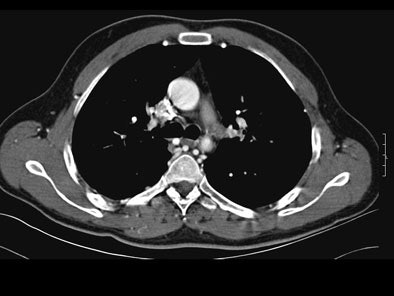
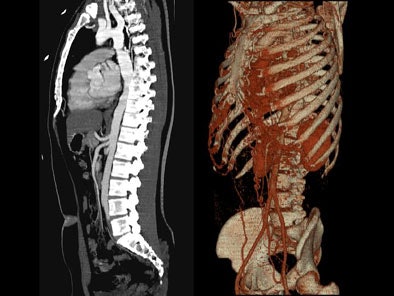
On postcontrast axial CT images, there was significant narrowing of descending thoracic aorta distal to the origin of left subclavian artery, associated with adjacent collaterals and hypertrophied internal mammary arteries. [Figure 2]
 |
| Figure 2. |
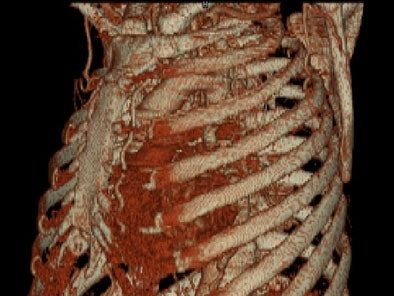
On sagittal MIP images, the morphology of coarctation along with a captured left subclavian artery was demonstrated. Volume-rendered images showed hypertrophied internal mammary arteries and epigastric arteries. [Figures 3, 4, 5] [Movie 1]
 |
| Figure 3. |
 |
| Figure 4. |
 |
| Figure 5. |
|
|
| Movie 1. |
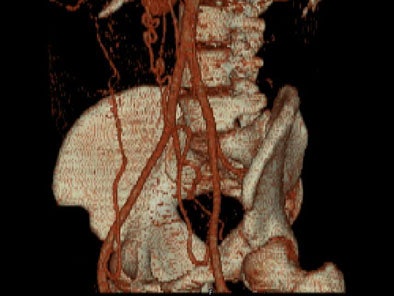
Zoomed oblique volume-rendered images displayed the coarctation of the aorta, distal to the left subclavian artery. [Figure 6] [Movie 2] [Movie 3]
 |
| Figure 6. |
|
|
| Movie 2. |
|
|
| Movie 3. |
Coronal volume-rendered images displayed the hypertrophied internal mammary arteries and intercostals arteries. [Movie 4]
|
|
| Movie 4. |
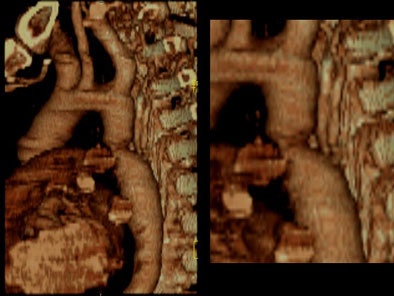
On virtual angioscopy, an endoluminal perspective of the circumferential infolded shelf was generated. An increase in number and size of intercostal collaterals, below the level of coarctation was evident. [Movie 5]
|
|
| Movie 5. |
An elective stenting/angioplasty has been advised, and the patient is under follow-up.
Discussion
Coarctation is a term derived from the Latin "coarctation", which refers to pressing together or narrowing. Coarctation is essentially a congenital obstructive anomaly of the aortic lumen, commonly located at the aortic isthmus, between the left subclavian artery and the ductus [1].
The overall imaging strategy in a given case of coarctation of the aorta comprises of analysing three distinctly important groups of findings: a) morphological description of coarctation, b) mapping the various collaterals in thorax and abdomen, and c) identifying associated anomalies of cardiovascular and other systems. Of late, MDCT and MRI have augmented the diagnostic armamentarium in delineating the coarctation as well as collateral circulatory pathways [1] [2] [3] [4].
For a better understanding of evaluation of coarctation of the aorta by MDCT, it is important to dwell on few fundamental areas like pathophysiology, collaterals pathways, rib notching, and conditions associated with coarctation of the aorta.
The pathophysiological hallmark in coarctation is a defect in the aortic media that produces an infolded and involuted shelf along the circumference of the aortic lumen [5]. The ductus (patent embryonic remnant) or ligamentum arteriosus (fibrosed remnant) is located usually at this level, and differentiates coarctation as pre- or postductal. Consequent to the presence of infolded shelf into the aortic lumen, stenosis develops, which impedes the blood flow, resulting in development of collateral arterial pathways, which connect the proximal and the distal aspects of the aorta.
Broadly, the principal groups of collaterals (seen in movies 1 and 4) are:
Thoracic collateral pathways that comprise the thoracoacromial and descending scapular arteries (from subclavian arteries) that supply the poststenotic descending thoracic aorta with retrograde flow via the intercostal arteries.
Thoracoabdominal collateral pathways that comprise the internal mammary arteries (from subclavian arteries) that connect not only with the descending thoracic aorta via the intercostal arteries, but also with the external iliac arteries via the superior and inferior abdominal epigastric arteries.
Specifically, the subclavian artery assumes an important role through its principal branches by distributing blood into anastomotic channels as outlined below [1]:
Via internal mammary artery, intercostal arteries to postcoarctation descending thoracic aorta.
Via thyrocervical and costocervical trunks to thoracoacromial and descending scapular arteries to postcoarctation descending thoracic aorta.
Via vertebral artery to anterior spinal artery to intercostal arteries to postcoarctation descending thoracic aorta.
Regarding rib notching in coarctation of the aorta, the following facts have been well established.
Roesler's sign is an eponymous term denoting notching of the ribs.
Notching is essentially due to pressure erosion by tortuousity of the intercostal arteries.
The underlying basis of rib notching is the presence of an intercostal circuit that is created by anastamosis of hypertrophied posterior intercostal with anterior intercostal arteries. The anterior intercostals are branches of internal mammary artery, while posterior intercostals are branches of thoracic aorta. Together they complete an intercostal circuit that enables blood flow to bypass the segment of aortic coarctation.
Inferior rib notching is rarely seen before age 5 years, but seen commonly in adults.
Rib notching is usually confined to the posterolateral portions of the ribs, in view of increased tortuousity of posterior intercostals as compared to anterior intercostals.
Notching is commonly confined to the third to the ninth ribs, since the intercostal circuits are complete at these levels.
It is absent in the first and second ribs because the upper two intercostal arteries do not participate in collateral circuits, arising from the thyrocervical trunk that is located proximal to coarctation.
They are not present in lower ribs below the ninth rib, due to the incomplete length of the lower ribs.
The notching is right-sided in preductal coarctation and left-sided in coarctation associated with an anomalous right subclavian artery [5].
By delivering low pressure blood to right axilla and arm, the anomalous right subclavian artery cannot support a collateral circulation with reversed flow through the right intercostal arteries.
Associated cardiovascular abnormalities in aortic coarctation include a) bicuspid aortic valve (up to 85%), b) berry aneurysms at the circle of Willis (3% to 5%), c) anomalous origin of the right subclavian artery (5%) [6], d) involvement of the left subclavian artery in the coarctation, and e) ventricular septal defect. Noncardiac abnormalities can involve genitourinary tracts and respiratory, gastrointestinal, and musculoskeletal systems [1] [2] [13]. Turner's syndrome is a condition frequently associated with coarctation, in a phenotypic female.
From the above discussion, it becomes increasingly clear that MDCT has the capability of comprehensively evaluating coarctation of aorta because it offers valuable information on all three distinct groups of findings: a) morphology of coarctation, b) mapping the collaterals, and c) identifying associated anomalies.
Few practical points emerge regarding technique used in evaluation of coarctation of the aorta. The coverage of scan should extend from the root of neck to the upper thigh, to enable adequate demonstration of the various groups of collaterals. Optimal contrast study is mandatory necessitating the use of pressure injector, automated triggering software, and large-bore 18-gauge catheter that delivers substantial contrast volume. Biphasic study, as done in this case ensures sustained opacification of femoral artery, whose branches participate in collateral formation. Further refinements that have occurred more recently has been the application of prospective ECG-gated MDCT scans [6].
Concerning the common rendering techniques available, the following broad points come forward in the evaluation of coarctation of the aorta:
Multiplanar reformation (MPR) is the simplest 3D technique that utilizes stacked axial sections to generate an imaging volume, and is useful for delineation of coarctation morphology.
Shaded surface display (SSD) is cumbersome and needs correct threshold selection and cumbersome editing to generate opaque surface representations of aorta.
Maximum intensity projection (MIP) is particularly useful for sagittal display of coarctation of the aorta. It displays only the brightest voxel along a line from the viewer's eye through the image, with darker voxels in front or behind not displayed, and does not use any threshold selection [7].
Volume rendering (VR) is the preferred method for 3D postprocessing, with each voxel within the dataset characterised by brightness, color, and opacity that forms a comprehensive vascular image of aorta. By manipulating transfer function and trapezoids and by flexible use of clip planes, interactive rotation and zoom, VR provides valuable images of coarctation of the aorta, collaterals, rib notching, and postsurgery anastomotic shunts [2] [6].
Virtual angioscopy offers "on-the-fly" endoluminal view of coarctation of aorta and collaterals. Cine angioscopic images can be generated from MDCT and MRA datasets [8].
Finally, two relevant issues in imaging coarctation of aorta are the entity "pseudocoarctation of the aorta" and the type of surgical and interventional options available at the moment.
Pseudocoarctation of the aorta is a rare, congenital anomaly, often asymptomatic and characterized by stenoses, or kinking or buckling of the descending thoracic aorta distal to origin of the left subclavian artery [9]. Described first by Souders in 1951, it differs from true coarctation by the absence of significant hemodynamic obstruction [10].
In view of the increasing use of MDCT in evaluating coarctation of the aorta, before and after surgical correction [6], an outline of the various interventional and surgical techniques available today assumes importance. The interventional options include balloon coarctation angioplasty and stent placement [11] [12]. The surgical options available are a) interposition graft, b) end-to-end anastamosis, c) prosthetic patch onlay grafts/patch aortoplasty, d) arch augmentation, e) jump graft bypassing the aortic coarctation segment, and f) subclavian flap aortoplasty [13].
By Surg Cdr I.K. Indrajit, Surg Cdr N. Mahesh, Surg Capt J. DSouza, and Col M.N. Sree Ram
AuntMinnieIndia.com contributing writers
December 1, 2004
The authors are from the departments of radiodiagnosis and cardiology at Indian Naval Hospital Ship Asvini, Mumbai, and the department of radiodiagnosis at Armed Forces Medical College, Pune.
References
- Sebastià C, Quiroga S, Boyé R, Perez-Lafuente M, et al. "Imaging of the Aorta. Aortic Stenosis: Spectrum of Diseases Depicted at Multisection CT." RadioGraphics 2003;23:S79-S91. Available at http://radiographics.rsnajnls.org/cgi/content/abstract/23/suppl_1/S79. Accessed on 12 Nov 2004.
- Cademartiri F, Nieman K, Raaijmakers RH, Alfieri O, Krestin GP. "Three-dimensional volume rendering with multislice computed tomography in the evaluation of aortic coarctation." Ital Heart J 2003; 4: 286-7. Available at http://www.italheartj.org/pdf_files/20030085.pdf. Accessed on 12 Nov 2004.
- Haramati LB, Glikstein JS, Issenberg HJ, Haramati N, Crooke GA. "MR imaging and CT vascular anomalies and connections in patients with congenital heart disease: Significance in surgical planning." RadioGraphics 2002; 22:337-349. Available at http://radiographics.rsnajnls.org/cgi/content/full/22/2/337. Accessed on 12 Nov 2004.
- Pitlick PT, Anthony CL, Moore P, Shifrin RY, Rubin GD. "Three-dimensional visualization of recurrent coarctation of the aorta by electron-beam tomography and MRI." Circulation 1999; 99: 3086-7.
- Davies M and Guest P J. "Pictorial review: Developmental abnormalities of the great vessels of the thorax and their embryological basis." BJ Radiology 2003 76, 491-502. Available at http://bjr.birjournals.org/cgi/content/full/76/907/491. Accessed on 12 Nov 2004.
- Cademartiri F, Mollet N, Nieman K, Alfieri O, Krestin GP. "Motion-free ECG-gated 16-row multislice computed tomography in the follow-up of aortic coarctation with three-dimensional volume rendering." Ital Heart J 2004; 5 (2): 167-168. Available at http://www.italheartj.org/pdf_files/20040064.pdf. Accessed on 12 Nov 2004.
- Schaffler GJ, Sorantin E, Groell R, et al. "Helical CT angiography with maximum intensity projection in the assessment of aortic coarctation after surgery." AJR Am J Roentgenol 2000; 175:1041-1045. Available at http://www.ajronline.org/cgi/content/full/175/4/1041. Accessed on 12 Nov 2004.
- Glockner JF. "Navigating the aorta: MR Virtual Vascular Endoscopy." RadioGraphics. 2003; 23:e11-e11. Available at http://radiographics.rsnajnls.org/cgi/content/full/23/2/e11. Accessed on 12 Nov 2004.
- Kessler RM, Miller KB, Pett S, Wernly JA. "Pseudocoarctation of the aorta presenting as a mediastinal mass with dysphagia." Ann Thorac Surg 1993; 55:1003-1005.
- Maziarz DM, Koutlas TC. "Coarctation and Interrupted Aortic Arch: Surgical Perspective." eMedicine. Available at http://emedicine.com/ped/topic2824.htm. Accessed on 12 Nov 2004.
- Thanopoulos BD, Hadjinikolaou L, Konstadopoulou GN, et al. "Stent treatment for coarctation of the aorta: Intermediate term follow up and technical considerations." Heart 2000; 84(1): 65-70.
- Saba SE, Nimri M, Shamaileh Q, et al. "Balloon coarctation angioplasty: Follow-up of 103 patients." J Invasive Cardiol 2000; 12(8): 402-6.
- "The Canadian Cardiovascular Society's Consensus conference 2000 update: Recommendations for The Management Of Adult Patients with Congenital Heart Disease." Available at http://www.ccs.ca/society/conferences/archives/2001/2001coneng-02.asp. Accessed on 12 Nov 2004.
Copyright © 2004 AuntMinnie.com