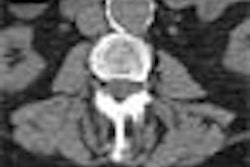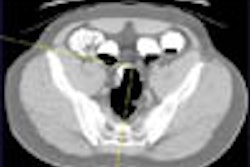
Colorectal cancer statistics speak volumes. In chapter one, for example, we learn that CRC is the second-leading cause of cancer death in the U.S., with a cumulative lifetime risk of about 6%. About 55,000 Americans die each year of the disease, mostly because fewer than 40% of adults have ever been screened.
"The challenge is really not to come up with a better test in terms of validity or effectiveness in sensitivity, specificity, and predictive value," said Dr. Paul Schroy, associate professor of medicine at Boston University School of Medicine. "The challenge is to come up with a valid test that is acceptable to patients, convenient, safe, inexpensive, and accessible."
Schroy offered his views on colorectal cancer screening during an April presentation at the International Symposium of Virtual Colonoscopy in Boston. He was one of two brave gastroenterologists (that is, people who do real colonoscopy) to address a meeting thick with radiologists (who tend to prefer their exams virtual). The second gastroenterologist to take the stage, Dr. Robert Schapiro from Massachusetts General Hospital in Boston, said he felt like Charlton Heston at a gun-control rally.
But gunslinging aside, the two specialties were brought together for a reason: to weigh the best available information about the detection and treatment of colorectal cancer, and use it to craft a screening strategy for the future. A successful screening strategy will need to improve disease outcomes by making the best use of emerging technologies, scarce dollars, and a wealth of imperfect options.
The process begins with tough questions, such as which screening test should be performed when and on whom? What cost and outcomes data are available or still needed? Should polyps smaller than 5 mm be removed, reported, followed up? If so, who will do all the follow-up colonoscopies and polypectomies? Which screening approach saves the most lives for the least money? And finally, will patients accept a cost-effective solution?
There's no need to reinvent the wheel. While guidelines vary somewhat, U.S. medical groups generally recommend a fecal occult blood test (FOBT) annually after about age 50, plus flexible sigmoidoscopy every 5 years, plus something like colonoscopy or double-contrast barium enema (DCBE) every 10 years. But new technologies such as virtual colonoscopy and fecal DNA testing are changing the paradigm. Schroy spoke of stool-sample testing for DNA abnormalities that could potentially spot any aerodigestive cancer.
"We know there are multiple abnormalities in stool-specific genes and oncogenes which would allow us to look for markers of transformation," he said. "DNA is shed continuously in the stool in exfoliated cells, it's stable in stool, and there's no bowel (prep) or medications necessary, unlike the fecal occult blood test."
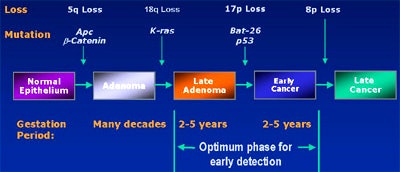
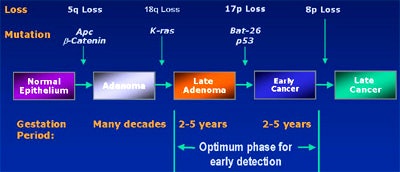
Because there is a 2-5-year window to pick up such mutations before invasive cancers develop, such an assay test could be an ideal screening method. The trick is to actually pick up a mutation, he said. The first of three fecal DNA trials to be conducted so far, the EXACT trial, suggests that it's possible to detect transformed cells containing mutations such as the K-ras oncogene, the APC tumor gene, or the p53 tumor suppressor, in as little as 6 grams of stool.
 |
| Colorectal cancer model courtesy of EXACT Science. |
"We found that only transformed cells seem to have this capacity to exfoliate long DNA. So it's unique to transformation; it's not part of normal cell apoptosis," he said. "Another important feature of stool testing is that it's not specific to the colon. Any organ system that enters the digestive tract has the potential for this kind of testing," Schroy said.
The EXACT trial assayed 17 genetic markers. In 22 patients with known colorectal cancer, the stool test was 91% sensitive for cancers (20/22), and 82% sensitive for adenomas (9/11), with a specificity of 93% (Gastroenterology, November 2000, Vol. 119:5, pp. 1219-1227).
Last year at Baltimore’s Johns Hopkins University, a study conducted on behalf of the U.S. National Cancer Institute looked for three mutations, including p53, K-ras, and the Bat-26 mononucleotide repeat marker. The study sought fewer markers than the EXACT trial, and perhaps as a result, had lower sensitivity: 71% for the overall detection of colorectal cancers, or 36 of 51 patients (Journal of the National Cancer Institute, June 2001, Vol 6:93, pp. 858-865).
In a study published this year, researchers at the MD Anderson Cancer Center in Houston and the Lahey Clinic in Burlington, MA, looked for just 1 marker. The APC oncogene had a sensitivity of 57% (26/46 patients), including 61% of Dukes B2 cancers (17/28) and 50% for adenomas larger than 1 cm, with specificity of 100% (NEJM, January 2002, Vol. 346:5, pp. 302-304).
The overall detection rate for the three tests was about 77%, Schroy said. "The problem here is that if you're screening for colorectal cancer you're going to pick up a lot of positives," he said. "If you don't want to do colonoscopy it lends itself to some interesting questions."
The future promises more and better tests, higher sensitivity and specificities, and perhaps even better biology, Schroy said. The tests can also be used to predict chemosensitivity or radiation sensitivity. A large multicenter trial is currently underway for individuals of average risk, 65 years or older.
Schroy 's future screening strategy includes flexible sigmoidoscopy plus stool DNA screening every 5 years, and colonoscopy or a VC exam (that's far more comfortable than the ones currently available) every 10 years.
Colonoscopy versus virtual, and the rest
"At age 50, between 20% and 50% of the population will have adenomatous polyps, but only a small percentage of these will go on to colon cancer," said Schapiro of MGH. "The guess would be anywhere from 1 to 20, and the question is whether one can predict who will. Ideally, we want to find colorectal cancer before it is acute and symptomatic."
No published studies have proven decreased colorectal cancer mortality from either colonoscopy or VC, though strong indications have been gleaned from old studies using fecal occult blood testing (FOBT) and colonoscopy, he said. Even though FOBT detects only 5% of polyps, 30% of localized cancers, and 50% of regionally invasive cancers, he said, three FOBT trials found a significant reduction in colon cancer deaths and a 20% decrease in new cancers. A flexible sigmoidoscopy trial found a 50%-70% decrease in colorectal cancer mortality even though cancers in the proximal colon, beyond the reach of the sigmoidoscope, were missed.
Schapiro cited a recent study that pegged the cost of performing colonoscopy twice in patient's lifetime at $20,000 per year of life saved. Performing it only once, at age 65, lowered the cost to just $2,000 per year of life saved, even if all the polyps were followed up (American Journal of Medicine, December 2001, Vol. 111:8, pp. 593-601).
"Now that's a very reasonable figure for a screening procedure, much better than for mammography or for cervical cancer," Schapiro said.
As for one gastroenterologist's view of VC, "we look at it as being an effective media for detecting medium and large-size polyps," he said. "It's certainly better than barium enema. But it has a downside in its requiring virtually the same preparation as colonoscopy, and there's a certain incidence of false-positive polyps. And we have in the back of our minds a little bit of concern because the current studies have been done basically on high-risk subjects, and have been done by academically invested groups."
Of course, colonoscopy's not perfect either, he said. One study showed that it missed 20% of tiny polyps, 13% of moderate-sized polyps, and 6% of polyps over 1 cm, only slightly better than VC.
A number of arguments have been advanced against choosing colonoscopy. "It's invasive, it's often incomplete, it's uncomfortable, it's too costly, and the social costs are greater because of patients losing time from work -- and that CT scans provide other diagnostic benefits," Schapiro said.
The risk of perforation in colonoscopy varies in the literature, from 1 in 2,500 to about 1 in 200. But it's important to remember that nearly all perforations occur in therapeutic procedures, including patients who are having relatively large polyps removed, or who are being examined because of ulcerative colitis or massive GI bleeding, he said. For elective colonoscopy, the perforation rate is about 1 in 3,000, even if a polyp is found. New technologies such as variable-stiffness colonoscopes have also improved the completion rate of traditional colonoscopy.
"We did a peer review of our own patients who did not have obstructing lesions, and we had a 96% success rate in getting to the cecum," Schapiro said.
Some studies indicate a patient preference for colonoscopy in a sedated state versus virtual colonoscopy in an unsedated state. Most VC protocols call for air insufflation to the maximum level tolerated by the patient, so it's clear that there will be some discomfort, he said.
Moreover, the cost of VC must be reduced to 54% of the cost of traditional colonoscopy, it is estimated, in order to compensate for follow-up in the case of positive findings. Right now the true costs of the procedures are actually comparable, he said, and are estimated at about $300 per patient per exam.
That's because VC uses expensive equipment, and expensive professional review, yet lower-cost assistance. Colonoscopy uses relatively cheap equipment, but the costs of recovery and professional monitoring after conscious sedation -- e.g., expensive assistance -- increase the total cost of the procedure, he said.
And with virtual colonoscopy the social costs are lower. The patient doesn't need a driver, and can return to work the same day. How many VC patients actually do go back to work the same day is a good question, Schapiro said.
"This is how I view (VC) in my practice. I think it's the best radiology study for colorectal cancer screening," he said. "I think it's a procedure of choice for default situations such as an incomplete colonoscopy, a patient who has had a difficult procedure in the past, or a patient who is just skittish about the concept of having a colonoscopy -- perhaps somebody who's relatively old and feeble, (for whom colonoscopy) is going to be a real stress. But I don't think it's ready for prime time as the screening procedure."
What would put VC into prime time? Schapiro wants a substantial cost decrease, reliable computer-aided detection (CAD), and perhaps an effective prepless exam combined with digital subtraction technology.
A radiologist looks at screening
Dr. Joseph Ferrucci, chairman of radiology at Boston University School of Medicine, also had some thoughts on screening strategies.
Regarding which procedure is more comfortable, he said, reported patient preferences are all over the map, and cover a wide range of exam protocols. In five abstracts and three papers he's looked at, all post-procedural questionnaires, the results were mixed. Some patients preferred conventional colonoscopy, some preferred virtual, and at any rate the VC methods were all outdated, he said. "There's nothing reported with comfort enhancers, self-insufflation with room air, or CO2."
An ideal screening strategy might be some sort of colon cancer diagnostic center, which would realistically be a hospital, he said. An onsite radiologist and endoscopist would work at opposite ends of the hallway. Virtual colonoscopy results would be read immediately, and positive cases referred for same-day colonoscopy. He acknowledged that the logistics of such a setup could be difficult, but said that with cooperation between the specialties, the scenario might be possible.
"We've learned that it's best to wait a couple of hours so the gas can be expelled, so that the endoscopist is more likely get to the cecum," he said. "We could help our friends in endoscopy by sending them a hard-copy printout or an e-mail" including standard (cm) measurements of the polyp's distance from the anus.
Even so, variables that will affect any proposed screening strategy will need to be addressed, he said. These include polyp target size, the costs and risks of the particular approach, and the interval at which the procedure is performed. The goals and aggressiveness of the chosen screening strategy must also be defined.
No existing trials have been done with prepless or computer-aided detection technology, he said, so new ones are needed for these factors too, with the outcomes measured.
"What do we need to prove? What level of evidence does this new technology have to reach? Cost-benefit? Years of life saved? I think it's fair when we think about the evidence levels and standards to ask ourselves what we're going to compare VC with in terms of the certainty that FOBT, flexible sigmoidoscopy, and other (techniques) have demonstrated in terms of saving lives. Not that we're not going to strive for it. But I don't think we should be overly timid in pursuing strategy at the national level, and with the carriers to compare ourselves in VC with these other techniques."
For the future, a plausible screening strategy might consist of initial screening with DNA markers, followed by virtual colonoscopy for the detection and localization of polyps, followed by colonoscopy when it becomes appropriate to remove them endoscopically, he said.
Final comments
From the audience, gastroenterologist Dr. Theodore Levin said he and his colleagues can't do all the colonoscopies that need to be done, and that the virtual colonoscopy alternative is needed just to handle the volume. While Schapiro schedules VC after failed colonoscopy, Levin said that at his institution, incomplete colonoscopies are generally rescheduled. Levin also questioned the wisdom of looking for extracolonic findings, and advocated using the lowest possible radiation dose for VC instead.
Scheduling too many follow-up colonoscopies is a major trap, Schapiro responded. The problem is that while very few polyps ever become advanced lesions, there is as yet no reliable way of predicting which ones will advance. He advocated removing both large and small polyps when they're found in the course of screening.
Schroy agreed, and said that as VC gets better at picking up smaller and smaller polyps, radiologists will be obligated to refer those patients for colonoscopy. By one estimate, 65% of the population has an adenoma, he said.
"So we're going to end up with colonoscopy for the entire population, we're going to have two tests (virtual and conventional colonoscopy), not just one. I'm happy with a (polyp) size cutoff, but it's going to require more frequent exams. So there's still a utilization issue and still a manpower issue."
Schapiro said follow-up at 5 or 10 years seems reasonable, but that doctors often face hysteria on the part of patients who have learned they have a polyp.
"And frankly," Schapiro said, "when faced with trying to explain to a patient, 'Well, you really don't have to be worried about this polyp,' and the patient saying, 'Yes, but I want to be sure I don't have any polyps,' or 'I had a little rectal bleeding and the internist said I'd better have the colonoscopy redone' -- the path of least resistance is often to redo it."
By Eric BarnesAuntMinnie.com staff writer
June 3, 2002
Copyright © 2002 AuntMinnie.com