Fujifilm Medical Systems USA of Stamford, CT, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its FDR D-Evo flat-panel digital radiography (DR) cassette.
The 14 x 17-inch cassette-sized detector weighs 6 lb and is designed for portability to address lateral or other cassette-based exams as needed. It also can be used as a complement in existing DR rooms, such as for cross-table exams.
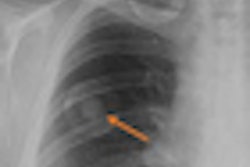
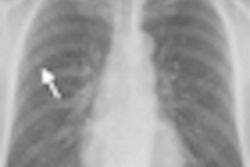
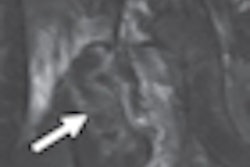
FDR D-Evo also uses Fujifilm's patented irradiation side sampling technology to enhance detective quantum efficiency (DQE) and produce images with enhanced sharpness and reduced noise for increased diagnostic confidence, according to the company.
Related Reading
Fuji folds endo unit into medical division, June 29, 2010
Mikron nets Fuji deal, Shimadzu install, June 21, 2010
Fuji to show new Fusion software, May 25, 2010
Fujitani to head Fuji in U.S., April 30, 2010
Fuji aids NFL draft prep, April 13, 2010
Copyright © 2010 AuntMinnie.com