A new first-time exhibitor at the RSNA meeting in Chicago earlier this month was Riverain Medical, a Dayton, OH, firm that is bringing to market its RapidScreen technology for computer-aided detection of lung nodules on radiographs. If RapidScreen sounds familiar, it's because it is -- RapidScreen was originally developed by Deus Technologies, a Rockville, MD, firm that Riverain bought in July 2004.
Deus appeared on the medical imaging scene in 2001, and quickly differentiated itself from other CAD firms by targeting the market for radiography-based lung CAD, rather than mammography. The firm received premarket approval (PMA) for its RapidScreen RS-2000 product in July 2001, and inked a deal with GE Healthcare in which the Waukesha, WI-based multimodality vendor offered RapidScreen as an option on sales of new digital radiography (DR) systems.
Fast forward to 2004. Riverain Medical was formed to acquire Deus by a pair of Ohio-based investors. The company has set up its headquarters and sales and marketing functions in Miamisburg, OH, while maintaining the Deus facility in Rockville as its R&D center.
Riverain is continuing the business strategy that Deus started by concentrating on lung CAD on radiography images, according to Stephen Sledge, vice president of business development at the company. While most of the attention in CAD has been concentrated on mammography or CT lung applications, Deus sees opportunity in the more than 7 million chest radiographs taken each year. Applying CAD to these exams could help physicians identify the nodules that could be precursors to lung cancer, he said.
"The premise is that because (the patients) have had the procedure done anyway, why not go ahead and run the radiography through the CAD system and see if there are any nodules in an early stage," Sledge said.
Riverain has modified the Deus strategy to include film-based radiography in addition to digital studies acquired by DR and computed radiography (CR) systems. Riverain's RapidScreen RS-2000 system for conventional radiography includes a digitizer for processing analog studies, while the RS-2000D model is designed to work with digital x-ray exams as part of a PACS network.
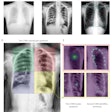
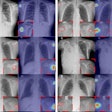
Like other CAD systems, RapidScreen works by applying image analysis algorithms to digitized images, with suspicious areas highlighted for further review on either a workstation or a paper printout. The company says the algorithm is based on pattern-recognition technology with fuzzy logic and neural networks.
Clinical studies conducted in support of RapidScreen's PMA indicate that the technology can help radiologists find 23% more cancers in the 9-14 mm size range than without CAD, Sledge said.
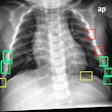
A study presented at the 2004 RSNA meeting found that when used alone on conventional x-ray images collected with a GE DR system, RS-2000D correctly identified the location of 21 of 37 solitary noncalcified nodules, resulting in a sensitivity of 57%. The researchers also used RS-2000D on energy-subtracted DR images, with the system correctly identifying 24 nodules for a sensitivity of 65%. When the results of the conventional and energy-subtracted images were combined, RS-2000D had a sensitivity of 81%.
Reducing false-positive results is a major issue in CAD, and the study found that when examining cases with a single solitary pulmonary nodule, RS-2000D had an average of 2.1 false-positive marks per image on conventional x-ray exams and 1.6 marks per image on energy-subtracted images. On cases without lung nodules or extensive interstitial disease, there was an average of 2.8 false-positives per image for conventional x-ray studies and 2.1 on energy-subtracted exams.
Riverain has assembled a network of x-ray dealers to sell RapidScreen, which will carry a list price of $150,000 for a basic unit on up to $200,000 for a system that has multiple DR and CR units plugged into it. GE continues to have rights to sell RapidScreen with new DR systems, and Riverain also plans to promote the technology through relationships with other CR and DR vendors.
Riverain doesn't plan to stop with radiography lung CAD, however. The company is developing new algorithms to analyze other lung diseases that are commonly detected first with radiography, such as tuberculosis and SARS. Such products could have particular application in developing countries where screening could find disease early, but where radiologists are in short supply, Sledge said.
By Brian Casey
AuntMinnie.com staff writer
December 20, 2004
Related Reading
Foundations laid, CAD technology builds mainstream support, January 13, 2004
GE, Deus unveil lung cancer CAD unit, July 8, 2003
GE, Deus to partner in lung CAD, November 26, 2002
Deus to use IP paper, November 6, 2001
Deus gets clearance for lung-cancer CAD, July 13, 2001
Copyright © 2004 AuntMinnie.com