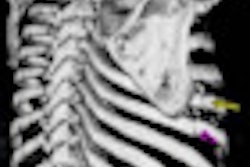
A team from the NIH and Carestream Health collaborated to develop the application and integrate it into the institution's PACS software. The tool allows localization of each lesion across time and also measures its longest diameter, short axis, and volume, according to presenter Dr. Les Folio of the NIH.
"With one click, [about 80% of] previous lesions are localized and measured, fully automatically," Folio said. "This results in a tremendous time savings."
In a study involving 19 metastatic melanoma patients with 71 lung, liver, and subcutaneous lesions, a majority of lesions were successfully and semiautomatically segmented and measured on baseline studies. They were also automatically identified, measured, and segmented on follow-up exams, Folio said.
"This means that once lesions have been identified [and] measured, when another radiologist, oncologist, or physician extender opens the follow-up exam to interpret, one button will find ... previous lesions, automatically measure, and export values to a [Response Evaluation Criteria in Solid Tumors (RECIST)] report," Folio said. "This may lead to more widespread use of RECIST by radiology departments to provide more quantitative reports to include tumor burden assessment."
Folio noted that tumor volumes were also obtained by the system, but they were not evaluated in this particular study.