Coronary Artery Calcification and Coronary CTA:
Clinical:
Basis for coronary calcium screening:
Pre-clinical detection of coronary artery disease (CAD) can result in early institution of risk modification. Clinical risk factors do not always identify patients with atherosclerotic CAD [28]. Assessment for the presence of coronary artery calcium is an excellent method for screening asymptomatic patients for the presence of CAD. The development of atherosclerotic plaque is accompanied by the deposition of crystals of hydroxyapatite (calcium phosphate) [45] and calcification does not occur in normal coronary arteries. Therefore, the presence of coronary artery calcification (CAC) indicates underlying coronary artery disease [9] and the amount of calcification correlates with the atherosclerotic plaque burden [24,45,83,100]. In one study, 35% of patients with a calcium score between 11-400 had significant coronary stenoses on CTA, and this increased to 65% of patients with a score above 400 [83].
CAC tends to be more common in complex plaques with hemorrhage and necrosis. The best use of CAC screening is to refine patient cardiac event risk assessment by providing incremental information over conventional risk factors [45,46]. A person with a likelihood of cardiac events of less than 10% per 10 years is considered low risk and is unlikely to benefit from additional cardiac testing [92]. For patients in an intermediate risk category (Framingham risk score 10-19% or 3 or more cardiac risk factors) coronary artery calcium scoring can provide incremental prognostic information regarding risk prediction for coronary events [28,29] and is cost effective in this population (which comprises about 40% of the general population [92]) [45]. Even in patients with normal myocardial perfusion exams, up to 17.5% can be shown to have coronary artery disease with calcium scores of greater than 100 [57]. Early identification of these patients can result in earlier medical intervention and risk factor modification [57].
Another group of patients that may benefit from early screening are patients with diabetes [68]. Patients with diabetes have a 4 times greater incidence of CAD than the general population [80]. Silent ischemic coronary artery disease can be found in 6-59% of these patients [68,119] and when CAD becomes clinically manifest it is often in an advanced state [80]. In general, diabetics have higher CAC scores compared to patients without diabetes (the likelihood of having a CAC score in the highest age/gender quartile is 70% greater for diabetics) [68]. Additionally, for every increase in CAC score, there is a greater increase in the mortality rate for diabetic patients than for patients without diabetes [68]. Measurements of CAC have been found to be superior to established cardiovascular risk factors in predicting silent myocardial ischemia and short term cardiovascular events in asymptomatic diabetic patients [119].
Patients with renal failure also have a higher incidence of CAD- in fact, CAD is the leading cause of death (50%) in patients with ESRD and this typically occurs within the first year of hemodialysis [70]. Early detection and treatment may result in improved outcomes [70].
Significance of exam findings:
A negative CT test makes the presence of atherosclerotic plaque very unlikely and is associated with a low risk for a cardiovascular event in the next 2-5 years [35]. Conversely, patients with CAC have an adjusted relative risk ratio for cardiac events 20 times higher than that for patients without CAC [29]. In general, the higher the calcium score, the greater the risk for cardiac events- in one study, the risk of cardiac death or non-fatal MI was 3.9 times higher for patients with calcium scores greater than 300, compare to patients with a calcium score of zero [28].Studies have also shown that the severity of coronary artery stenosis and frequency of myocardial infarction/events correlate with the amount of calcification [9,10,30,35]. Current evidence supports aggressive anti-atherosclerosis therapy in patients with calcium scores above 100 [46].
However, at what calcium score should further cardiac evaluation be performed? Because CAC has only a modest correlation with the degree of coronary lumenal stenosis, myocardial perfusion imaging should be considered in patients with high CAC scores to better define the clinical significance of the patients underlying coronary artery disease [45]. The presence of even moderate amounts of coronary calcium on CT (scores over 100) have been shown to be associated with silent ischemia in up to 18% of patients [45] and can predict an increased risk for cardiac events in symptomatic and asymptomatic individuals [24]. In general, the likelihood for a normal myocardial perfusion SPECT exam (MPS) decreases with increasing calcium score [83,100] and there appears to be a direct relationship of the extent and severity of perfusion abnormalities to the CAC score [95].
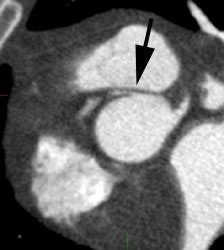
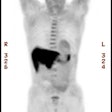
Coronary artery calcification: The patient below has a large calcification in their proximal LAD. Their total calcium score was 537. |
|
Myocardial perfusion imaging (MPS) has been shown to be normal in up to 87% of vessels with calcium scores of less than 10, and in 85% with scores between 11-100 [83]. For scores between 11-100, MPS will demonstrate evidence of ischemia in 2.6- 4.8% of patients [95]. For scores between 100-400, MPS will be abnormal in 5-18% of patients [95]. Generally, patients with a calcium score of greater than 400 are considered to be at the greatest increased risk for subsequent cardiac events [31]. In one study, 41% of asymptomatic patients with a calcium score of greater than 400 had abnormal MPS, whereas, no abnormal scans were observed in patients with scores of under 100 [29]. In general, the prevalence of abnormal MPS is between 15-46% for patients with calcium scores over 400 [45,95]. These results indicate that the likelihood of myocardial ischemia by SPECT is related to the coronary calcium score [46] and that it is appropriate to evaluate asymptomatic patients with CAC scores above 400 using MPS [95]. Bear in mind that many patients with scores above 400 still have normal MPS exams [83]. Importantly, data suggests that when patients with high CAC scores have normal MPS findings, their prognosis with aggressive medical treatment is good [45] with an annualized event rate as low as 1% [119]. Although a CAC score of 400 is a reasonable threshold to prompt MPS in patients with a low to intermediate likelihood for CAD, an important point to consider is that patients with a high clinical likelihood for CAD may require a lower CAC score threshold [106]. In patients with multiple CAD risk factors, MPS can be considered in symptomatic patients and even those with low calcium scores as up to 15% of patients with scores of less than 100 may have perfusion abnormalities on MPS [74]. Therefore, CAC screening and myocardial perfusion imaging should be considered complimentary examinations and should be used in conjunction with the patients clinical evaluation to determine the most appropriate management [45,74,106].
Although the actual calcium score is a good indicator of overall extent of disease, it may not be as useful for predicting cardiac events as a percentile ranking [24]. This is because coronary artery calcium generally increases with age [24]. A calcium score above the 75th percentile for age and sex matched individuals is associated with a much greater risk for cardiac events compared to individuals in the 25th precentile [24]. For instance, a calcium score of 40 in a 40 year old man would place him above the 95th percentile, while a 70 year old man with an identical score would be below the 10th percentile [24]. Patients in the top calcium score quartile have up to an 8-fold increased risk for cardiac events compared to patients in the lowest quartile [45]. Asymptomatic patients with calcium scores above 100 who are above the 90th percentile should also be considered for SPECT myocardial perfusion evaluation due to their increased frequency of silent ischemia [46]. However, although a high percentile ranking is indicative of long-term risk for cardiac events, the finding is not always associated with ischemia on perfusion imaging [77]. In fact, there is a low likelihood (2.5-7%) for identification of ischemia on perfusion imaging in patients with scores below 100 due to the sub-clinical level of atherosclerotic disease [77]. Hence, perfusion imaging may not be required if the calcium score is less than 100, despite a high percentile ranking [77]. This group of patients (high percentile score with CAC less than 100), however, can benefit from aggressive antiatherosclerotic intervention [77].
|
Calcium Score |
Recommendation |
| Less than 100 (but high percentile) | Does not necessarily require perfusion imaging (MPS). (+) Atherosclerotic intervention. |
| Percentile 90% or higher and score over 100 | MPS should be performed |
| Over 400 | MPS should be performed |
| Score 100-399 | Consider clinical factors: sex, chest pain sx's, diabetes and selective MPS evaluation |
Of course, coronary calcium score is just another variable to consider when evaluating a patient. Gender, smoking, and the presence of symptoms may modify a patients overall risk for cardiac events and the need for further cardiac evaluation [45].
Limitations:
Coronary calcium scoring cannot rule out coronary artery disease in all patients [77]. Although a calcium score of zero indicates a very low likelihood for significant coronary artery disease, it does not completely exclude the risk for cardiac events [28]. Critical single vessel CAD occurs in 2-5% of subjects with a negative CAC score [45] and approximately 5% of patients with acute myocardial infarction have no coronary artery calcium [77].. Middle-aged women with a smoking history appear to be particularly susceptible to having events in the absence of, or with minimal CAC [45]. In a study in the Journal of the American Medical Association, coronary events were observed in 4.4% of patients with calcium scores of zero during a mean follow-up of 7 years [28]. These events are likely related to rupture of soft, unstable lipid plaques [28,45]. However, calcium may not have been properly detected in all patients as this study employed a standard CT scanner and 6 mm slice thickness [28]. Retrospective cardiac gating, multidetector imaging, and thinner slices have all been shown to improve detection of coronary artery calcification [8,16,18,27]. CT coronary angiography can be used to detect the presence of soft plaque in these patients.
Serial Coronary Calcium Evaluation:
Patients with moderately high calcium scores (exceeding 100) should be treated aggressively with anti-atherosclerosis therapy [46,56]. Serial monitoring of the patient's calcium score can be used to assess effectiveness of medical therapy [46]. Evidence supports that the CAC scores can be reduced with aggressive lipid-lowering drug therapy (such as statins which will slow the propagation of vascular calcification) [46,47]. Treated patients show only a small progression in calcium score (percent change in volume score of 10% or less) and can even demonstrate a reduction in the calcium volume [46]. Conversely, untreated patients can demonstrate progressive coronary calcification with a 35-40% increase in calcium volume score [46]. Patients with higher baseline scores tend to progress at a faster rate than individuals with lower baseline scores [46]. Patients that demonstrate progressive coronary calcification with a change of greater than 30-40% are at high risk for subsequent myocardial infarction [46].
X-ray:
The chest radiograph has a low sensitivity for detecting coronary artery calcification. The best site to detect coronary artery calcification on the PA CXR is along the mid left heart border (the left main, and proximal portions of the left anterior descending and left circumflex arteries lie in this location). CAC appears as thin, parallel white lines on the chest radiograph. The accuracy of CXR for detecting coronary calcifications is only 42% [4]. Plain film evidence of coronary artery calcification in symptomatic patients under the age of 65y, is almost always (nearly 100% incidence) associated with significant coronary artery disease. About 30% of asymptomatic patients will also have significant narrowing.
Fluoroscopy has also been used successfully for the detection of coronary artery calcification. Sensitivity ranges from 40%-79% [4]. However, up to 48% of calcifications identified on EBCT can be missed at fluoroscopy [4].
Helical CT: For the detection of significant coronary obstructive disease, helical CT has a sensitivity between 88-91% and a specificity of about 50% (accuracy 76%) [4]. The quantity of coronary artery calcifications measured by helical CT correlates positively with obstructive CAD [2] and the absence of coronary calcification has a high negative predictive value for the exclusion of CAD in patients with atypical chest pain [6]. Although lack of calcification does not exclude atherosclerotic plaque (about 5-10% of patients have CAD despite the absence of coronary calcifications) [6] it does indicate a low likelihood for obstructive CAD. Calcific deposits are often blurred on single slice helical imaging because of cardiac motion and small calcifications may not be seen [4]. Retrospective ECG gating of diastolic data has been shown to improve detection of calcified coronary plaques (less cardiac motion during diastole) [6]. The coronary calcium scoring system used for electron beam CT may not be entirely applicable to helically acquired exams, but further investigation is required [14]. A more appropriate threshold for calcium may be 90 HU [16]. Note however that helical exams are more prone to streak artifacts from calcifications [16]. If these streaks are included when determining the coronary calcium score, the resultant score will erroneously high [16]. The estimated peak radiation dose from the exam is 2.7 rad at the skin (breast dose is 1.8 rad, lung dose 1.7 rad) [14].
The presence of CAC on pre-operative CT scanning is associated with cardiac complications during non-cardiac thoracic surgery, however, the positive predictive value is low (23%). The absence of coronary artery calcium has been shown to be a reliable predictor of a favorable post-op cardiac course [3].
Multisection helical CT:
A 16-slice scanner can obtain an entire cardiac scan in about 12 heartbeats which should be possible using a single breath hold [7, 33]. Because of its shorter acquisition time, superior spatial resolution (0.625 mm longitudinal which decreases partial volume artifacts [37]), and temporal resolution approaching that of EBCT, 16 slice MDCT is superior to EBCT for the evaluation of coronary artery disease [33]. 32 and 64 slice scanners offer the ability to acquire images in an even shorter period of time due to a larger field of view. For most patients the mean Agatston score is not significantly changed by using a 1.5 mm reconstruction increment, as opposed to the usual 3 mm increment [88]. However, in young patients, very small amounts of calcium may not be identified unless overlapping slice reconstruction is used [88]. In young patients, even small amount of coronary artery calcification can influence the estimation of their cardiovascular risk [88]. (Proposed imaging protocol)
MDCT interscan variability: As with all cardiac calcium exams, even multi-detector helical CT examinations suffer from interscan calcium score variability. Generally, variability is greatest for patients with minimal CAC and decreases with increasing amounts of CAC. Heart motion and partial volume averaging can account for a 10-50% (average about 30%) change in score (depending on lesion size) when patients are imaged twice within a short interval [24,53]. The percentile ranking assigned to the two scans can differ in up to 13% of patients (using 4-slice MDCT) [42]. To reduce interscan variability, thin slice imaging will help to reduce partial volume effects and overlapping reconstructions are also useful [53]. When using prospective gating for the exam, interacquisition variability in calcium measurements is significantly less at slower heart rates- likely due to decreased cardiac motion artifacts [21]. One way to reduce interscan variability is to repeat the exam in subjects who have a minimal to mild calcium score on the initial scan and average the two results [22]. Unfortunately, this will increase patient radiation exposure. The use of calcium volume measurements rather than a calcium score, has also been shown to decrease variability in the exam [22].
Retrospective ECG gating can be performed to decrease interscan variability and also improve the quality of the exam [35,53]. Using this technique, partially overlapping MDCT projections are continuously acquired and the ECG signal is simultaneously recorded [33]. Algorithms are then used to sort the data from different phases of the cardiac cycle into specific temporal windows (typically 10 windows) based upon the R-wave (i.e.: the R-R interval is divided into 10 equal time windows) [33]. Through the use of retrospective ECG-gating, the point in the cardiac cycle at which cardiac motion is minimal (typically late diastole) is used for image reconstruction producing excellent multiplanar reformatted images [7,13]. Multi-detector exams with retrospective gating have been reported to have less inter-exam variability (to about 10% or less [35,53]) when compared to EBCT studies [18], however, other authors have found persistent high interscan variability [26].
Retrospective ECG gating does not rely on estimation of the presumed next R-R interval as is seen in prospective gating [13]. Reconstruction data intervals can be accurately measured on the basis of the actual R-R intervals that occurred during the scanning [13]. The final reconstruction is based only on images obtained during a portion of mid-diastole, with the reconstruction trigger selected according to the actual heart rate (and this greatly reduces cardiac motion artifacts) [13]. The fastest and easiest image reconstructions use a pre-defined window of 40% of the RCA and 60% for the LAD [26]. However, using preselected reconstruction intervals can result in under-estimation of the true calcium score (and calcium volume)- particularly for patients with mild or moderate amounts of coronary calcium [37]. Improved image quality can be obtained by reconstructing several image sets and selecting the data with the fewest motion artifacts for calcium score determination [26,37]. For multi-detector helical CT acquisitions there is an inverse relationship between heart rate and image quality [13]. Overall, the best image quality is achieved when the patients heart rate is less than 75 beats per minute [13]. Reconstruction image quality decreases with higher heart rates, irregular heart rates, or arrhythmias [13].
Further analysis has shown that each of the individual coronary arteries are best seen at varying points of the cardiac cycle [15]. The LAD at mid-diastole (50-70%), the circumflex at 50-60%, and the right coronary artery at 40-50% [14,15]. The left coronary artery, aortic root, and ascending aorta are best seen at mid-diastole (50-60%) [39]. Detection of small calcifications in the coronary arteries is improved when images are viewed at different reconstruction points [66]. In fact, up to 58% of patients with a calcium score of zero at one reconstruction interval, were found to have coronary artery calcifications when multiple reconstructions were reviewed [66]. The pulmonary arteries are best displayed during mid-to late diastole (80%) [39].
Even with these variations, multidetector CT with retrospective ECG-gating produces cardiac images with fewer motion artifacts and shows a high correlation with coronary artery calcium scores determined using electron beam CT [16].
The one major drawback of retrospective gating is that it increases the patients radiation exposure compared to prospective ECG triggering due to oversampling [16]. Radiation dose from multidetector coronary CT can vary widely depending on the type of imaging protocol used from 1.5 to 5.2 mSv for male patients, and from 0.6 to 6.2 mSv for female patients [20,22,23]. The radiation exposure from spiral protocols using retrospective gating is at least 3 fold higher than that from electron beam CT [20]. Studies have shown that lower radiation doses can be used for the exam- this results in images with an increased amount of noise, but apparently detection of calcification is not greatly affected [19,23]. The patient dose can be reduced by lowering the mAs (to as low as 40-55 mAs) without significantly compromising exam quality, particularly for non-obese patients [22,60].
One other point to consider is that variation in attenuation values can affect CAC determination (which depends on the detection of aggregates of contiguous image pixels with attenuation values greater than 130 HU) [48]. Image attenuation values have been shown to vary between scanners and by body size [48]. This can produce calcium score values that do not reflect the true calcium burden [48]. Calibration phantoms containing known amounts of calcium hydroxyapatite can improve accuracy and permit comparability of measurements between persons imaged on different scanners [48].
Overall, EBCT and MDCT have been shown to have equivalent reproducibility for measuring calcium scores [58].
Electron beam CT (EBCT): EBCT is capable of very rapid scans which can be triggered from prospective ECG gating (generally at 80% of the R-R interval) to minimize cardiac motion [4]. The estimated peak radiation dose from the exam is between 1.1 to 1.3 rad (cGy) and no contrast material is required [4,14]. Unlike a nuclear medicine exam, no exercise or pharmacologic stress agent is required for the exam. An Agatston scoring scale is used to determine risk of cardiac events [24]. Using this method the slice thickness should be 3 mm and a Hounsfield density measurement greater than +130 is considered indicative of calcification [4,24]. For each level scanned, all pixels with a HU density measurement greater than 130 HU are then displayed and a region of interest (ROI) is placed around the lesion [9]. If using a more narrow slice thickness, a mathematical correction must be applied to avoid oversampling [24]. For instance, if the slice thickness were 2.5 mm, the appropriate scaling factor would be 2.5/3 [24]. To be considered for the final score determination a calcification needs to have a minimum area of greater 1 mm3 [8]. A lesion density score is then determined based upon the maximal HU number in each ROI [9]- 1= 130 to 199; 2= 200 to 299; 3= 300-399; and 4 is greater than 400 HU. This lesion density score is then multiplied by the area of each region of interest to determine a calcium score [9]. The total calcium score is determined by adding up each of these scores from each of the four coronary arteries (left main, left anterior descending, circumflex, and right coronary arteries) [9,11]. A total calcium score of 0 is normal; 1-100 considered mild coronary artery disease; 101-400 moderate; and over 401 indicates extensive coronary artery disease and very high risk for cardiac event [30]. Up to 18% of asymptomatic patients with calcium scores between 100-399 will have perfusion abnormalities indicative of ischemia on stress myocardial perfusion SPECT imaging [36]. The higher the coronary artery calcium score, the more likely patients will have an abnormal myocardial perfusion SPECT exam (abnormal scans demonstrating ischemia are found in 45% to 46% of patients with scores above 400) [17,30,36].
Scans performed at 3 mm slice thickness are superior to those performed at 6 mm for accurate determination of coronary artery calcium score [8]. Even more accurate determinations of calcified volume can be obtain by using 1.5 mm thick slices [27]. The extent of calcification has a high correlation with a significant stenosis somewhere in the involved coronary vessel and the greater the extent of calcification, the more severely narrowed the coronary artery is likely to be [4].
Coronary artery calcification also carries prognostic information as the greater the amount of calcification identified- the more likely the patient is to have a cardiac event [4,10]. The annualized event rate for patients with calcium scores greater than 400 can be as high as 14% [11]. Additionally, in patients with significant coronary artery stenoses, the presence of CAC is associated with a worse prognosis [1]. Unfortunately, a single standard score to define abnormal is unlikely to be applicable to all ages and both sexes [5]. Clinical decisions should not be based solely on the coronary artery calcium score [5]. Other factors such as individual risk factors and medical history need to be reviewed [5].
There can be considerable variability in EBCT coronary calcium measurements when two scans are performed on the same patient in close temporal proximity (between 14-50%) [4,5,26]. Interscan variability is most pronounced for patients with low, but non-zero calcium scores [5]. Interscan variation is an important limitation of electron beam CT and patients may require two scans with the higher calcium score used for risk stratification [5]. The interscan variability is likely related to scan misregistration, cardiac motion, breathing, and inherent image noise [4,12,22]. Scanning the entire heart during one breath hold will resolve the problem of scan misregistration [12].
Interscan variabilities can also be decreased by the use of an ECG trigger of 40% of the R-R interval (rather than 80% which is the trigger used for many EBCT exams) [12]. This is because coronary artery motion is at its nadir during early diastole (the period of 30-50% of the R-R interval), while the 80% trigger occurs on or near the P-wave during atrial systole [12]. Images obtained at the end of systole or at early diastole (beginning at 10-30% of the cardiac cycle) also suffer from motion artifact [13]. For prospective triggering of EBCT, the next R-R interval is estimated on the basis of the median of the last seven R-R intervals [13]. Prospective ECG triggering fails with rapid changes in heart rate, as in the case of patients with cardiac arrhythmias [13].
The sensitivity of electron beam CT for coronary artery disease has been reported to be between 88-100% [16] and the overall specificity is about 50%. The low specificity is due to the fact that CAC confirms the presence of plaque disease, but it may not be hemodynamically significant. Absence of CAC implies the absence of a significant coronary artery narrowing and is associated with a very low risk for future cardiac events (annual event rate less than 1%) [4,11]. The negative predictive value of an normal EBCT exam for significant stenosis (ie: 50% or greater) is between 90-95% [4]. However, lack of CAC does not confirm the absence of coronary artery disease- particularly in younger patients [4]. EBCT can be used in the evaluation of patients presenting to the emergency department with chest pain and non-diagnostic ECG's (excluding patients with prior MI or revascularization procedure) [11]. If the patient has a normal EBCT exam the risk of immediate MI is very low and the patient does not necessarily require admission [11]. Unfortunately, patients without visible calcium may still be experiencing an acute coronary syndrome [84].
In patients with no measurable coronary calcification at baseline, a followup EBCT score must exceed 11.6 Agastone units to qualify for statistically significant progression [63].
CT coronary angiography:
Patients with low to intermediate risk factors who present with atypical chest pain would benefit from a noninvasive test with a high negative predictive value [92]. Although coronary calcium score can aid in the detection of CAD in asymptomatic patients, stenoses can occur in vessels which do not contain calcification [49]. Additionally, recent studies have indicated that calcification in plaque is an indicator of stable plaque [62] and that calcification is found infrequently in ruptured plaque [105]. Ideally, it is the non-calcified or vulnerable plaque that would be most useful to identified. CT angiography can provide additional insight into the presence of CAD by permitting visualization of non-calcified plaque and has been shown to be more accurate than calcium scoring in demonstrating coronary stenoses [49]. Conventional angiography yields only information about luminal diameter- not about plaque characterization [62]. Multidetector CT (MDCT) offers several other advantages for coronary artery evaluation- the exam is non-invasive, it can be completed in a short period of time, myocardial bridging can be readily appreciated, and it can effectively define coronary anomalies [46,54]. MDCT can also be performed for evaluation of stenosis of coronary artery bypass grafts [85].
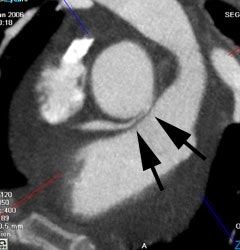
Anomalous RCA origin from the left coronary sinus: The patient below underwent coronary CT angiography to assess for coronary artery disease. The patient was found to have an anomalous RCA arising from the left sinus of valsalva. The vessel can be seen to course between the pulmonary trunk and aorta (black arrows) |
|
Limitations:
The most significant drawback of the CT exam is in it's suboptimal ability to consistently detect the presence of anatomic coronary lesions [46]. In general, visualization of the epicardial coronary arteries is limited by cardiac motion, the small size of the distal arteries, and the tortuous course of the vessels through the imaging plane [34,99]. The right coronary artery has the greatest amount of motion and velocity- generally between 6-42mm of axial displacement during the cardiac cycle (3-20mm for the left coronary artery) [99]. These factors are important because of the limited temporal and spatial resolution of the CT scanner. Temporal resolution is limited to one-half the gantry rotation speed (this is about 200-300 msec for most state of the art scanners) while angiography can capture 30 frames per second (approximately 30 msec temporal resolution). The spatial resolution is limited by the size of the CT detectors (presently with over sampling the resolution is about 0.5 mm). Therefore, CT coronary angiography generally has very good sensitivity for stenoses in the proximal and mid-segments of the main coronary vessels (vessels over 2 mm in size) [69], but the evaluation of the distal main vessels and their side branches is much more limited [69]. The current generation of 64 slice scanners have an in plane resolution of 0.4 mm, a slice thickness of 0.6 mm, and a temporal resolution of 165 ms [71,75]. Temporal resolution can be improved with:
Multisegment reconstruction: For patients with stable and predictable heart rates, the temporal resolution can be further improved to as low as 83 msec by applying a multisegmental reconstruction algorithm [75,99]. With multisegment reconstruction, data of two or more successive cardiac cycles are combined that cover, as separate segments, a 180 degree acquisition [99]. The temporal resolution is improved by a factor 2n (n=number of cycles and segments) of the rotation time [99]. However, multisegment reconstruction can result in motion artifact if the heart rate is not constant [75].
Dual Source CT: Dual source CT is characterized by two x-ray tubes and two corresponding detectors mounted on the gantry with an angular offset of 90 degrees [97,99,113]. Thus, a 90 degree rotation of the gantry is sufficient to acquire the 180 degree projection data needed for image reconstruction [113] and this improves the temporal resolution by a factor of 2 [97,99]. With a gantry rotation time of 330 msec, dual source CT has improved temporal resolution (83 msec by half-scan reconstruction) and this may decrease the need for pharmacologic heart rate modulation [89,97,99]. It may also be useful for the evaluation of patients with atrial fibrillation [109]. Motion free imaging of the coronary vessels is possible even at heart rates of up to 90 bpm [113]. Only heart rates that are both high and variable deteriorate image quality with dual source CT [97]. Faster gantry rotations are not possible at this time due to an increase in mechanical G-forces that are beyond mechanical engineering limits [99]. For dual source CT, the overall optimal reconstruction window has been suggested to be at 75% of the R-R interval (for patients with low or intermediate heart rates) [113]. In patients with heart rates above 80 bpm, systolic reconstructions may yield superior image quality [113].
Another limitation of CT coronary angiography is related to partial volume averaging of coronary artery calcification which results in calcifications appearing larger than their actual size [34].
Finally, it is important to remember that not all coronary stenoses identified by CT are flow limiting lesions [69]. Patient management based solely upon the CT findings can lead to inappropriate revascularization [69]. Myocardial perfusion imaging should be strongly considered in patients with CT lesions to determine their functional significance [69]. Non-flow limiting lesions probably warrant aggressive medical therapy [69].
Contraindications:
1- High density objects: Calcified coronary plaques and metallic cardiac stents can affect the quality of the exam [69]. Dense/large calcifications obscure the true vessel lumen due to blooming effects when the calcification partially extends into adjacent voxels [69]. Therefore, CT angiography may not useful in patients with a heavy burden of calcified plaque (score greater than 400-1000) which precludes adequate evaluation of the degree of stenosis [50,71]. However, other authors have found no significant effect in patients with high calcium scores [86]. Also, photon flux through high density objects can sometimes lead to a signal void adjacent to a densely calcified plaque or within a stent which can be misinterpreted as soft plaque [69]. The use of higher spatial frequency algorithms can aid in stent evaluation.
2- Arrhythmias: Patients with atrial fibrillation or other forms of irregular heart rhythms are also not candidates due to suboptimal image quality [50,102]. Patients with mild heart rate irregularities may undergo imaging, but may require ECG editing to improve image quality [67].
3- Patients with breath-hold difficulties and those with the inability to remain supine and motionless are also not likely good candidates for CT coronary imaging [50].
4- Limited diagnostic accuracy has been reported in obese patients with a body mass index of 35 kg/m2 or greater [102].
Other exclusion criteria include known allergy to iodinated contrast, underlying renal dysfunction (serum creatinine greater than 1.36 - 1.5 mg/dL), multiple myeloma, hyperthyroidism, pheochromocytoma, and patients with acute coronary syndromes [52,71].
Patient preparation:
With use of 16 to 64 detector scanners, noninvasive CT imaging of the coronary arteries for non-calcified plaque is done by making adjustments to keep the patients resting heart rate low and utilizing retrospective gated reconstructions [32,33,34]. For coronary CT angiography, image quality is inversely related to the patients heart rate (i.e.: image quality declines with increasing heart rate) [35]. The right and circumflex coronary arteries seem more prone to motion artifact than the LAD- possibly related to their proximity to the atria which is reactivated during early diastole [43]. Motion-free coronary angiograms can be obtained in the majority of patients with heart rates below 80 beats per minute [40], however, the slower the heart rate the better the image quality. Heart rate variability also affects image quality with less variable heart rates producing better quality exams [82]. This is because the commonly applied relative ECG-gating reconstruction technique (at a certain percentage of the R-R interval) does not generate images in exactly corresponding cardiac phases when there is intercycle variablity in the heart rate [82].
In general, it is desirable to have the patients heart rate less than 60 (generally between 50-59 bpm). β-blockers should be used to achieve the desirable heart rate if there are no contraindications (such as asthma, COPD, or bronchospasm on inhalers, sinus bradycardia [HR less than 60 bpm], hypotension [systolic BP less than 100 mmHg], atrioventricular conduction block (second or third degree block), heart failure, diabetes, and Raynaud syndrome) [35,50,72,92]. Caution should be used for patients who are taking other atrioventricular nodal blocking agents such as calcium channel blockers (diltiazem, verapamil), digoxin, and other β-blockers [72].
Oral propranolol (20-40mg) or metoprolol [Lopressor] (50-100mg) given the night before and 1 hour prior to the exam can also reduce heart rate and improve image quality (especially for right coronary artery visualization) [43,50,51]. Metoprolol can also be administered I.V., but requires that patients be monitored (HR and BP) carefully during dosing. The initial dose should be 2.5 mg I.V. over one minute [72]. If the heart rate remains above 65 bpm after 5 minutes, an additional dose of 2.5 mg is administered [72]. If the heart rate still remains elevated, up to two additional 5 mg doses can be given (each over one minute) with a 5 minute interval between doses [72]. A total of up to 15 mg of metoprolol can be used I.V. [35,50,72,92]. If the patient has bronchospasm, two puffs of an albuterol inhaler are given [72]. If the patients heart rate drops to less than 45 bpm, consideration is given to administering atropine [72]. If the patient is atropine resistant, resuscitative measures with IV fluids and epinephrine may be required [72].
Calcium channel blockers can be used to reduce the heart rate in patients with a contraindication to beta-blockers (such as congestive heart failure or asthma) [71,78]. Calcium channel blockers can be administered intravenously (diltiazem 0.25 mg per kg of body weight up to 25 mg maximum; or in an oral regimen of 30 mg of regular-release diltiazem) [92]. Sublingual administration of short-acting nitroglycerin (1 to 2 tablets equal to 0.4 to 0.8 mg) given immediately prior to scanning has been used to improve visualization of the coronary lumen (via the vasodilatory effect) [71,92,116]. Nitroglycerin has been found to increase proximal coronary artery diameters by 12-21% [99] and allows more septal branches to be visualized [116], but the added value on diagnostic accuracy is not yet clear [99]. Contraindications to nitroglycerin include hypotension, migraine sensitive to nitrates, recent MI, severe anemia, increased intracranial pressure, known hypersensitivity to nitroglycerin, and recent use of nitrate-based medication for erectile dysfunction [92,93].
All patients that receive medication for the exam must be monitored for 30 minutes follwoing the test and must not operate machinery or drive for 3 hours following the IV administration of 10 mg or more of metoprolol [92].
Scan Protocol and Radiation Dose:
Generally- scanning should be in a cranial-to-caudal direction- beginning at the carina and extending through the base of the heart during a breath hold [50] Valsalva maneuver should be avoided as this can result in poor contrast enhancement [99]. If bypass grafts or internal mammary arteries are to be evaluated, scanning should begin at the level of the aortic arch [50]. The thinnest detector collimation possible should be used for image acquisition [50]. An initial non-contrast exam is performed to determine the extent of coronary artery calcification which, if severe, will prevent CT angiography [50]. Between 60-120mL of a contrast agent with a high concentration of iodine (300-400 mg/mL) is administered via an 18 to 20-gauge catheter at a rate of 4-6 mL/sec [35,50,71,92,117]. The injection should be followed by a saline flush (25-50 mL at 4-6 mL/s) to decrease beam-hardening artifact within the right ventricle which can obscure the RCA [35,50,71,117]. Alternatively, contrast can be injected in a bi-phasic pattern with 50mL at 4mL/sec and 30-50mL at 2.5 mL/sec followed by a saline flush [51] . Another protocol calls for a biphasic injection with the second phase consisting of a 50 mL mixture of 30% contrast and 70% saline that is then followed by a saline flush [92].
The scan delay can be determined using a test injection (20 mL) with repeated scanning at 2 sec intervals positioned at the level of the aortic root [92]. Scan delayed can be empirically timed to coincide with the beginning of the saline flush (about 25 seconds) or bolus tracking can be performed with an ROI over the ascending aorta [50]. Bolus tracking may yield better vessel enhancement than a test bolus [38]. In general, contrast materials with higher iodine concentrations yield significantly higher attenuation in the coronary arteries [59].
Prospective ECG triggered exam: For this exam, instructions are built into the protocol to start imaging at a desired distance from the R-R peak- for example at 60% or 70% of the R-R interval [101]. The scanner, in congruence with the patient's ECG pulse, starts the scan at the preset point in the R-R interval [101]. The projection data are acquired for only part of the complete gantry rotation (i.e.: a partial scan) [101]. The table is then moved to the next location for further data acquisition (i.e.: this is a non-helical acquisition) [101]. The cycle is repeated until the entire scan length is covered- typically 12-15 cm [101]. The advantage of prospective triggering is reduced radiation exposure because the projection data is not acquired throughout the cardiac cycle [101,118]. [118].
A retrospectively ECG gated acquisition is the preferred method for contrast enhanced imaging of the coronary arteries [35]. Retrospective ECG gating coronary CT requires a highly overlapping spiral scan (typical pitch is 0.2 to 0.4) with a table speed adapted to the patients heart rate and simultaneous recording of the ECG trace which is used for linkage of scan data with particular phases of the cardiac cycle [35,101]. In other words, data is acquired throughout the cardiac cycle with a continuous helical acquisition and then retrospectively, projection data from selected points within the R-R interval are selected for image reconstruction [101]. Retrospectively gated studies can be reconstructed with partial scan data or with segmental reconstruction [101]. For partial scan reconstruction, the scan data required for reconstruction are obtained by rotating the x-ray tube 180 degrees plus the fan angle (30-60 degrees) of the CT detector assembly [101]. Therefore, the temporal resolution will be slightly greater than one-half of the gantry rotation time [101]. In multiple segmental reconstruction, the scan projection data to perform a partial scan reconstruction are selected from various sequential heart cycles, instead of from a single heart cycle [101]. In other words- data from different parts of the heart cycle are chosen, so that the sum of these segments equates to the partial scan data required for image reconstruction [101]. This results in improvement in temporal resolution [101]. The disadvantage is that because projection data sets are obtained from different heart beats, a mis-registration due to rapid motion can result in degradation of image spatial resolution [101].
Radiation exposure: A drawback of retrospective ECG gated CT is that it is associated with a higher radiation dose to the patient [35] because of overlapping data acquisition and acquiring data during cardiac phases that do not contribute to image reconstruction [118]. With a multi-detector row CT the radiation dose is approximately 7 to 10 mSv and is higher in women [35,50,52]. This dose is higher than the dose from conventional angiography (about 3-5 mSv) [50,51,78]. The radiation dose can be decreased by use of "ECG gated dose modulation" which uses the normal tube output during diastole, but reduces tube output during phases of the cardiac cycle that are of less importance for the ECG gated reconstruction (such as during systole) [35,50,71]. Depending on the heart rate, an overall exposure savings of 30-50% can be achieved using dose modulation without decreasing image quality (overall dose from the exam can be decreased to as low as 4 mSv and generally to 6.7-7.6 mSv in men, and 8.1-9.2 mSv in women) [35,51,52,78]. Further decreased radiation exposure can be achieved by decreasing the kilovoltage setting to 80 kV in slim patients [73]. ECG gated dose modulation is limited to use in patients with low (under 65 beats), steady heart rates [35,92]. One drawback of dose modulation is image noise in those portions of the cardiac cycle in which the lower exposure was used. This can sometimes limit evaluation of the cardiac valves and cardiac vessels (at other time points).
The radiation from CT imaging "may" pose a risk for increased incidence of breast and lung cancer [111]. One point to remember is that the excess lifetime risk of radiation induced cancer mortality declines significantly with age and that the majority of patients being evaluated for coronary artery disease are often elderly [98,111]. The excess relative risk for lung and breast cancer after a single multidetector CT of the chest is generally below 1% for individuals aged 55 years or older [111]. However, the lifetime excess risk for breast or lung cancer in girls and young women aged 15-25 years that undergo a single ECG-gated CT angiographic exam is higher- ranging from 1.7% to 5.5% for a single examination [111]. The risk for radiation induced cancer would be increased in an additive manner if multiple followup exams were performed [111].
Obese patients pose a special challenge for cardiac imaging as the high level of noise associated with the exam degrades both spatial and contrast resolution [92]. However, patients with more subcutaneous fat may actually have lower organ doses due to absorption of more of the photons in the subcutaneous tissues [111].
Exam processing:
The exam should be reconstructed with a slice thickness of 0.75-1mm with 25-50% overlap and a pixel matrix of 512x512 [50,71]. The image is reconstructed using data acquired during approximately 240? of the gantry rotation (not 360? ) [50]. This has the effect of improving the temporal resolution to about half of the gantry rotation time [50]. Cardiac CT image quality is determined less on the basis of spatial resolution than on the basis of temporal resolution (the time required for acquisition of each transverse image) [64]. For motion artifact-free image quality, the average duration of the R-R cycle must allow for 165-250 msec of cardiac akinesia [64]. At low heart rates this is easily achieved by placing the reconstruction interval in mid-diastole [64].
Diastole consists of 4 distinct phases: isometric relaxation, early rapid filling, diastasis (the myocardium is relaxed and the atrial and ventricular pressures have equalized), and atrial systole [113]. Image reconstruction in cardiac CT is typically performed in mid-to-late diastole during the period of diastasis [113]. Although diastasis is relatively long in patients with low heart rates, it shortens with increasing heart rate and even ceases to exist at heart rates of around 80 bpm [113]. Systole consists of 3 phases- isometric contraction, rapid ejection, and reduced ejection [113]. In contrast to diastole, the duration of systole is less affected by changes in heart rate [113]. The isometric contraction phase is initiated by the QRS complex and during this phase cardiac motion is small- unfortunately, the duration is too short to permit imaging [113]. Thus- only the phase of reduced ejection can be used for image reconstruction (this phase does decrease with increasing heart rate) [113]. One problem with with using systole for image reconstruction is image noise if dose modulation was utilized for image acquisition [113].
If available, a preview function that displays images at fixed R-R increments (every 5 or 10 %) should be used to determine the phase that depicts the fewest motion artifacts [92]. If a preview function is not available, then the first image reconstruction can be performed at 60% of the R-R interval [92]. For patients with higher heart rates (greater than 70 beats per minute) image reconstruction cannot be completed in the short diastolic time available without producing artifacts [50]. In these cases, multi-segmented reconstruction algorithms that utilize data from more than one cardiac cycle are required for data processing [34,50]. Image reconstruction is started from a specified delay from the prior R-wave (expressed as a percentage) [50]. Reconstructions at 10% increments from 40-80% will likely provide optimal images to evaluate all the vessels, however, additional reconstructions can be performed [50]. RCA motion is mainly determined by contraction of the right atrium [82]. Reports suggest the RCA is best evaluated during early diastole at 40% of the R-R interval (range 30-60% R-R interval while the right atrium is relatively motionless) [50,82]. Motion of the left circumflex (LCX) follows the left ventricle and the best reconstruction interval is during mid-diastole when the left ventricle is quiesent (50-70% of the R-R interval) [50,82]. The LM and LCA also follow left ventricular motion and are best seen at 60-70% of the R-R interval [50]. As discussed previously, in patients with high heart rates, the best image quality can be obtained with end-systolic and early-diastolic intervals [64]. For patients with high or irregular heart rates, a reconstruction window positioned in late systole (25-35% of the R-R interval) will often yield good image quality [71] (other authors suggest 40-45% of the R-R interval [113]).
Images are typically reconstructed with a medium smooth reconstruction kernal [71]. Sharper reconstruction kernals can be used for evaluation of coronary stents [71]. Larger field of view 3 mm images are reviewed to assess for incidental non-cardiac findings [71].
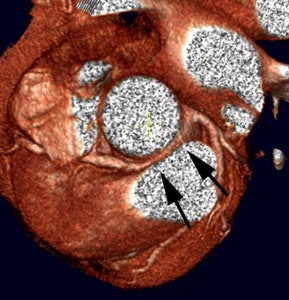
3D cardiac coronary angiography: 3 dimensional color image from a coronary CT angiogram. |
|
Results:
On MDCT, between 6-12% (up to 32%) of coronary segments are poorly assessable due to poor image quality (usually the result of cardiac motion or coronary artery calcification) [49,50,51,52,55]. The most frequently non-assessable segment is the middle right coronary artery [49,52]. Stent-bearing segments may not be well assessed due to artifacts and partial volume effects which prohibit reliable visualization of the coronary lumen [52].
Artifact: Artifact (black arrow) degrades evaluation of a section of the right coronary artery. |
|
Significant stenoses are considered greater than 50%, and high grade more than 70-75% [50]. Commonly, stenoses of less than 50% are revascularized only in the presence of clinical symptoms, whereas stenoses of more than 70% are ordinarily treated even in the absence of symptoms [93]. Lesions should be characterized by location, length, and severity, and associated plaque or calcification should be described [50]. Patent internal mammary arteries should be documented [50]. Comparison of MDCT with coronary angiography for coronary stenoses exceeding 50% has found a sensitivity of 73-95%, a specificity of 86-94%, a positive predictive value of 68-80%, and a negative predictive value of 97-99% [33,35,46,51]. However, other studies have indicated lower values- sensitivity 68% and specificity 99% for evaluable segments (with 43% of segments not assessable) [46]. However, the technology is rapidly developing and these numbers may not accurately reflect the current capabilities of CT coronary imaging [46].
Compared to 16 slice CT, 64 slice imaging results in a shorter exam times (9-13 seconds compared to 20 sec) and has improved temporal and spatial resolution that permits better analysis of more distal branches [110]. 64-slice CT imaging has been shown to have higher specificity and PPV compared to 16 slice imaging [110]. On 64 slice MDCT, up to 89-95% of segments can be assessable [75,76,99,120]. For significant (greater than 50%) stenosis, 64-slice MDCT has a sensitivity of 76-97%, a specificity of 79-98%, positive predictive value of 91-93%, and a negative predictive value of 83-96% [75,89,93,96,99,110,120]. However, most studies have had a high prevalence of obstructive CAD and this can falsely elevate sensitivity data [120]. Results are best for assessment of the most relevant, larger and mid coronary segments (greater than 1.5 mm) [89,120] and most studies do not include data from smaller or uninterpretable vessels [120]. Heavy calcification in the area of stenosis can result in over-estimation [93]. The sensitivity and specificity may be lower in patients with a body mass index of greater than 30 kg/m2 [79]. From a clinical perspective, a normal CTA is helpful because it effectively excludes the presence of obstructive CAD, defines a low clinical risk, and makes management decisions straightforward [120].
Coronary CT angiography: The left image is from a normal RCA. The right image shows hard (black arrow) and soft (white arrow) plaque in the LAD. |
|
Combining CT angiography with calcium scoring results in an overall improvement in sensitivity without a loss of specificity [49]. In a prospective study of 103 patients for the evaluation of significant coronary lesions (greater than 50%), the segment based sensitivity, specificity, PPV, and NPV of MDCT were 95%, 98%, 87%, and 99%, respectively [52]. The article concluded that MDCT provides highly accurate and non-invasive detection of suspected obstructive coronary artery disease [52].
Soft plaque: The patient below had a soft plaque (white arrow) producing an approximately 50% stenosis in the left circumflex coronary artery. Soft plaques such as this will not be identified without the use of IV contrast.. |
|
Functional significance of coronary stenoses detected on MDCT:
In general, there is good correlation between a negative coronary CTA exam and myocardial perfusion imaging (86% of patients with a normal exam will have normal perfusion imaging findings [PPV 86%]) [83]. However, despite the excellent negative predictive value there is a discrepancy between the anatomic extent of CAD and the presence of resultant ischemia [83,94,112]. Hence, although MDCT is capable of identifying morphologically significant stenoses, it cannot predict the functional significance of the lesion as it is unable to assess coronary collateral flow and vasomotor tone [55,79,91]. In one study, 53% of stenoses greater than 50% were not associated with a perfusion defect on perfusion imaging (positive predictive value of CT imaging for reversible defects was 29%) [55]. In another study, 51% of coronary vessels with stenoses between 50-90% had normal MPS exams [83]. Overall, the positive predictive value of CTA for identifying ischemia producing lesions (by SPECT) is between 29-44% [94,120]. Therefore, myocardial perfusion imaging will generally be required to assess the functional relevance of MDCT detected stenoses [55,79]. However, one can look at this from another perspective- that is to say that despite the presence of coronary artery stenoses over 50%, MPS failed to identify CAD in 51-53% of those vascular territories [55,83]. By identifying CAD on the CTA exam, these patients can be placed into aggressive medical treatment and life style modification [83,112]. Even in the absence of ischemia, knowledge of the presence of coronary atherosclerosis is important for patient management [56] and CTA can detect coronary artery disease at an earlier stage than MPS [83,112].
The perfusion and CT data sets can also be fused to form a 3-dimensional display and this can provide additional information regarding the hemodynamic significance of a coronary stenosis compared to side-by-side analysis (the radiation exposure of a 99Tc-perfusion agent exam and a cardiac CTA study is 15-25 mSv [1.5-2.5 rad]) [90].
Coronary CT findings and prognosis:
Patients with normal coronary CT exams have an excellent prognosis and are at very low risk for cardiac events [102]. Patients with significant stenoses are at a markedly increased risk for cardiac events and the event rate is also increased in patients with atherosclerotic disease, but without significant (less than 50%) stenoses [102]. Plaque type is also predictive of coronary evnt risk, with an increased risk associated with mixed plaques [102].
Coronary stent assessment:
Coronary stent restenosis occurs due to neointimal hyperplasia and is seen in less than 10% of drug eluting stents and up to 40% of uncoated metallic stents [104,107]. A lumenal diameter reduction of more than 50% due to neointimal hyperplasia is consistent with a hemodynamically significant stent restenosis [104]. Cardiac stents are associated with "blooming" artifacts due to their metallic content that results in artificial narrowing of the lumen of the stent [103]. Stent lumen visibility varies with stent type and diameter [87]. The blooming effect is more pronounced with smaller stents (diameter 3 mm or less) or those with thicker struts (over 140 ?m) [87,103]. Gold or gold-coated stents and those made of tantalum cause the most severe artifacts, while those made of stainless steel and cobalt are better visualized [87]. Another factor that degrades evaluation is partially overlapping stents- likely related to the increased metal content [103]. Partial volume averaging (CT averages the attenuation of materials within a voxel) also limits stent evaluation - particularly in small vessels [107].
To decrease stent associated blooming artifact, sharper reconstruction kernals (edge-enhanced high spatial resolution) are used for evaluation of coronary stents [71,87,92,107]. The use of the sharper kernal increases image noise, but allows better visualization of the stent [87]. In general, between 7-14% of stents are not assessible [102,103,104]. For the evaluation of in-stent restenosis or occlusion, 64-slice CT had a sensitivity of 89-97%, a specificity of 88-95%, a PPV of 77.5-94%, and a NPV of 90-98.5% [87,104]. In another study evaluating in-stent stenosis using 64-slice MDCT, 93% of stents with a diameter of 2.5 mm of greater could be evaluated with a sensivitity of 95%, and a specificity of 93% [102].
MDCT in acute chest pain:
MDCT coronary angiography has also been studied in the evaluation of patients presenting with an acute chest pain syndrome with promising results [65,81]. The exam results can aid in decreasing the number of unnecessary hospital admissions [81].
Coronary artery bypass graft evaluation:
Evaluation of coronary bypass grafts can be performed due to the large diameter of the vessels and the fact that they show considerably less motion during the cardiac cycle [102]. 64 slice CT can be used for accurate exclusion of greater than 50% graft stenosis or graft occlusion with a sensitivity of 85-97% and a specificity of 95-97% [98,102]. Detection of distal anastomotic stenosis is limited [98] as is evaluation of the small diameter native coronary vessels distal to the bypass graft [102].
Artifacts:
Cardiac motion is identified when there is a "stair step" artifact in the cardiac or vessel contour on reconstructed images (but not involving other structures such as the diaphragm) [50]. Since reconstruction is performed at a specified point from the R-wave, an aberrant R-wave that occurs sooner than expected (shortening the anticipated R-R interval) can result in the addition of systolic data and artifact to the reconstructed images [50]. By using ECG editing, the data set can be corrected by rejecting these beats [67]. With respiratory or patient motion, a "stair step" artifact will affect both the heart and the other structures such as the diaphragm and osseous structures [71].
"Blooming" artifacts occur from high attenuation structures such as calcified plaques or stents [71]. The artifact is a result of partial volume averaging and it can obscure the adjacent vessel lumen [71]. Sharper filters and thinner slices (0.5-0.6 mm) may reduce the artifact associated with stents, but do not effect artifact associated with calcification [71]. Also, photon flux through high density objects can sometimes lead to a signal void adjacent to a densely calcified plaque or within a stent which can be misinterpreted as soft plaque [69].
Functional cardiac analysis:
Functional cardiac analysis (wall motion and ejection fraction) can also be performed [33]. However, because a beta-blocker is used for MDCT imaging, the myocardial contractility may be affected and this may limit the utility of functional analysis [89]
MDCT multiplanar reformats of retrospectively ECG-gated acquired images permits evaluation of left ventricular function [44]. The ejection fraction, end diastolic volume, and LV mass estimated by multi-detector row CT correlate well with MR determined values [41]. Segmental reconstructed images can help to decrease motion related artifacts by improving temporal resolution [41]. Remember that the use of a beta blocker prior to CT imaging can result in changes in LV function and this can decrease the reliability of the measured parameters [41].
LV Values [108]:
| Variable | Value |
| EF | 69 +/- 6% |
| End-diastolic volume | 150 +/- 31 mL |
| End-systolic volume | 47 +/- 15 |
| Stroke volume | 104 +/- 21 |
| Mass (g) | |
| Men | 123 +/- 21 |
| Women | 96 +/- 27 |
RV values [108]:
| Variable | Value |
| EF | 61 +/- 6% |
| End-diastolic volume | 173 +/- 39 mL |
| End-systolic volume | 69 +/- 22 |
| Stroke volume | 104 +/- 21 |
| Mass (g) | |
| Men | 41 +/- 8 |
| Women | 35 +/- 7 |
Coronary MRA:
In general, the image quality of MRA is inferior to that of 64-MDCTA as a result of limited spatial resolution and increased motion artifact from longer imaging times [115]. However, MRA may have better diagnostic performance for the detection of significant stenosis in coronary artery segments with moderate to severe calcified plaque [114].
REFERENCES:
(1) Radiology 1980; 137(3): 609-616
(3) J Comput Assist Tomogr 1997; 21(4) 619-622
(4) Radiologic Clinics of North America 1999; Stanford W, Thompson BH. Imging of coronary artery calcification. Its importance in assessing atherosclerotic disease. 37(2): 257-272
(5) AJR 2000; Yoon HC, et al. Interscan variation in coronary artery calcium quantification in a large asymptomatic patient population. 174: 803-809
(6) AJR 2000; Becker CR, et al. Imaging of noncalcified coronary plaques using helical CT with retrospective ECG gating. 175: 423-24 (No abstract available)
(7) Radiology 2000; Ohnesorge B, et al. Cardiac imaging by means of electrocardiographically gated multisection spiral CT: Initial experience. 217: 567-571
(8) AJR 2000; Callister T, et al. Sensitivity of two electron beam tomography protocols for the detection and quantification of coronary artery calcium. 175: 1743-1746
(9) J Am Coll Cardiol 1990; Agatston AS, et al. Quantification of coronary artery calcium using ultrafast computed tomography. 15: 827-32
(10) Am J Cardiol 2000; O'Malley PG, et al. Prognostic value of coronary electron-beam computed tomography for coronary heart disease events in asymptomatic populations. 85: 945-948
(11) J Am Coll Cardiol 2001; Georhiou D, et al. Screening patients with chest pain in the emergency department using electron beam tomography: A follow-up study. 38: 105-110
(12) Radiology 2001; Mao S, et al. Effect of electrocardiogram triggering on reproducibility of coronary artery calcium scoring. 220: 707-711
(13) Radiology 2001; Hong C, et al. ECG-gated reconstructed multi-detector row CT coronary angiography: Effect of varying trigger delay on image quality. 220: 712-717
(14) Radiology 2001; Goldin JG, et al. Spiral versus electron-beam CT for coronary artery calcium scoring. 221: 213-221
(15) Radiology 2001; Kopp AF, et al. Coronary arteries: retrospectively ECG-gated multi-detector row CT angiograpgy with selective optimization of the image reconstruction window. 221: 683-688
(16) AJR 2001; Horiguchi J, et al. Quantification of coronary artery calcium using multidetector CT and a retrospective ECG-gating reconstruction algorithm. 177: 1429-1435
(17) Circulation 2000; He ZX, et al. Severity of coronary artery calcium by electron beam computed tomography predicts silent myocardial ischemia. 101: 244
(18) Radiology 2002; Kopp AF, et al. Reproducibility and accuracy of coronary calcium measurements with multi-detector row versus electron beam CT. 225: 113-119
(19) Radiology 2002; Hong C, et al. Coronary artery calcium measurement with multi-detector row CT: in vitro assessment of effect of radiation dose. 225: 901-906
(20) Radiology 2003; Hunold P, et al. Radiation exposure during cardiac CT: effective doses at multi-detector row CT and electron beam CT. 226: 145-152
(21) Radiology 2003; Hong C, et al. Coronary artery calcium quantification at multi-detector row CT: influence of heart rate and measurement methods on interacquisition variability- initial experience. 228: 95-100
(22) Radiology 2003; Takahashi N, Bae KT. Quanitification of coronary artery calcium with multi-detector row CT: assessing interscan variability with different tube currents- pilot study. 228: 101-106
(23) AJR 2003; Mahnken AH, et al. Detection of coronary calcifications: feasibility of dose reduction with a body weight-adapted examination protocol. 181: 533-538
(24) AJR 2003; Rumberger JA, Kaufman L. A rosetta stone for coronary calcium risk stratification: agatston, volume, and mass scores in 11,490 individuals. 181: 743-748
(25) Radiology 2003; Shaw LJ, et al. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. 228: 826-833
(26) AJR 2003; Van Hoe LR, et al. Coronary artery calcium scoring using ECG-gated multidetector CT: effect of individually optimized image-reconstruction window on image quality and measurement reproducibility. 181: 1093-1100
(27) Radiology 2003; Viegenthart R, et al. Coronary calcifcation at electron-beam CT: effect of section thickeness on calcium scoring in vitro and in vivo. 229: 520-525
(28) JAMA 2004; Greenland P, et al. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. 291: 210-215
(29) J Nucl Cardiol 2003; Moser KV, et al. Coronary calcium screening in asymptomatic patients as a guide to risk factor modication and stress myocardial perfusion imaging. 10: 590-598
(30) J Nucl Cardiol 2003; Cerqueira MD, Rumsey MP. Coronary calcium scoring: what does it really mean? 10: 692-695
(31) Radiology 2004; Stanford W, et al. Coronary artery calcium quantification at multi-detector row helical CT versus electron-beam CT. 230: 397-402
(32) AJR 2004; Hoffman MHK, et al. Noninvasive coronary imaging with MDCT in comparison to invasive conventional coronary angiography: a fast-developing technology. 182: 601-608
(33) AJR 2004; Desjardins B, Kazerooni EA. ECG-gated cardiac CT. 182: 993-1010
(34) Radiology 2004; Schoenhagen P, et al. Noninvasive imaging of the coronary arteries: current and future role of multi-detector row CT. 232: 7-17
(35) Radiology 2004; Schoepf UJ, et al. CT of coronary artery disease. 232: 18-37
(36) J Nucl Cardiol 2004; Anand DV, et al. Prevalence of silent myocardial ischemia in asymptomatic individuals with subclinical atherosclerosis detected by electron beam tomography. 11: 450-457
(37) Radiology 2004; Schlosser T, et al. Coronary artery calcium score: influence of reconstruction interval at 16-detector row CT with retrospective electrocardiographic gating. 233: 586-589
(38) Radiology 2004; Cademartiri F, et al. Intravenous contrast material administration at 16-detector row helical CT coronary angiography: test bolus versus bolus-tracking technique. 233: 817-823
(39) Radiology 2004; Hofmann LK, et al. Electrocardiographically gated 16-slice CT of the thorax: cardiac motion suppression. 233: 927-933
(40) Radiology 2004; Hoffmann MH, et al. Noninvasive coronary angiography with 16-detector row CT: effect of heart rate. 234: 86-97
(41) Radiology 2005; Yamamuro M, et al. Cardiac functional analysis with multi-detector row CT and segmental reconstruction algorithm: comparison with echocardiography, SPECT, and MR imaging. 234: 381-390
(42) AJR 2005; Halliburton SS, et al. Potential clinical impact of variability in the measurement of coronary artery calcium with sequential MDCT. 184: 643-648
(43) AJR 2005; Shim SS, et al. Improvement of image quality with beta-blocker premedication on ECG-gated 16-MDCT coronary angiography. 184: 649-654
(44) AJR 2005; Schlosser T, et al. Assessment of left ventricular parameters using 16-MDCT and new software for endocardial and epicardial border delineation. 184: 765-773
(45) J Nucl Cardiol 2005; Raggi P, Berman DS. Computed tomography coronary calcium screening and myocardial perfusion imaging. 12: 96-103
(46) J Nucl Cardiol 2005; Shaw LJ, et al. Computed tomography imaging within nuclear cardiology. 12: 131-142
(47) J Nucl Cardiol 2005; Miller DD, Shaw LJ. Screening for coronary heart disease: cardiology through the oncology looking glass. 12: 158-165
(48) Radiology 2005; Nelson JC, et al. Measuring coronary calcium on CT images adjusted for attenuation differences. 235: 403-414
(49) Radiology 2005; Lau GT, et al. Coronary artery stenoses: detection with calcium scoring, CT angiography, and both methods combined. 235: 415-422
(50) AJR 2005; Lawler LP, et al. MDC evaluation of the coronary arteries, 2004: how do we do it- data acquisition, postprocessing, display, and interpretation. 184: 1402-1412
(51) AJR 2005; Heuschmid M, et al. ECG-gated 16-MDCT of the coronary arteries: assessment of image quality and accuracy in detecting stenoses. 184: 1413-1419
(52) JAMA 2005; Hoffman MHK, et al. Noninvasive coronary angiography with multislice computed tomography. 293: 2471-2478
(53) AJR 2005; Horiguchi J, et al. Variability of repeated coronary artery calcium measurements by 16-MDCT with retrospective reconstruction. 184: 1917-1923
(54) Radiology 2005; Datta J, et al. Anomalous coronary arteries in adults: depiction at multi-detector row CT angiography. 235: 812-818
(55) J Nucl Med 2005; Hacker M, et al. Comparison of spiral mutlidetector CT angiography and myocardial perfusion imaging in the noninvasive detection of functionally relevant coronary artery lesions: first clinical experience. 46: 1294-1300
(56) J Nucl Cardiol 2005; Shaw LJ, Berman DS. Redefining the low-risk patient with signifncat atherosclerotic disease. 12: 375-377
(57) J Nucl Cardiol 2005; Thompson RC, et al. Clinical utility of coronary artery calcium scoring after nonischemic myocardial perfusion imaging. 12: 392-400
(58) Radiology 2005; Detrano RC, et al. Coronary calcium measurement: effect of CT scanner type and calcium measure on rescan reproducibility- MESA study. 236: 477-484
(59) Radiology 2005; Cademartiri F, et al. Intravenous contrast material administration at helical 16-detector row CT coronary angiography: effect of iodine concentration on vascular attenuation. 236: 661-665
(60) Radiology 2005; Shemesh J, et al. Coronary artery calcium measurement with multi-detector row CT and low radiation dose: comparison between 55 and 165 mAs. 236: 810-814
(61) Radiology 2006; Herzog C, et al. Multi-detector row CT coronary angiography: influence of reconstruction technique and heart rate on image quality. 238: 75-86
(62) AJR 2005; Dubinsky TJ. Coronary artery calcification scoring. 185: 1540-1541
(63) AJR 2005; Servukov AB, et al. Serial electron beam CT measurements of coronary artery calcium: has your patient's calcium score actually changed? 185: 1546-1553
(64) Radiology 2006; Herzog C, et al. Multi-detector row CT coronary angiography: influence of reconstruction technique and heart rate on image quality. 238: 75-86
(65) AJR 2006; Ghersin E, et al. 16-MDCT coronary angiography versus invasive coronary angiography in acute chest pain syndrome: a blinded prospective study. 186: 177-184
(66) AJR 2006; Sandstede JJW, et al. Different reconstruction intervals for exclusion of coronary artery calcifications by retrospectively gated MDCT. 186: 193-197
(67) AJR 2006; Cademartiri F, et al. Improving diagnostic accuracy of MDCT coronary angiography in patients with mild heart rhythm irregularities using ECG editing. 186: 634-638
(68) J Nucl Cardiol 2006; Scholte AJH, et al. Screening of asymptomatic patients with type 2 diabetes mellitus for silent coronary artery disease: combined use of stress myocardial perfusion imaging and coronary calcium scoring. 13: 11-18
(69) J Nucl Cardiol 2006; Di Carli MF, et al. Integrated cardiac PET-CT for the diagnosis and management of CAD. 13: 139-144
(70) J Nucl Cardiol 2006; Okwuosa T, et al. Coronary artery disease and nuclear imaging in renal failure. 13: 150-155
(71) J Nucl Med 2006; Hoffmann U, et al. Coronary CT angiography. 47: 797-806
(72) AJR 2006; Pannu HK, et al. β-blockers for cardiac CT: a primer for the radiologist. 186: S341-345
(73) AJR 2006; Abada HT, et al. MDC of the coronary arteries: feasibility of low-dose CT with ECG-pulsed tube current modulation to reduce radiation dose. 186: S387-390
(74) J Nucl Cardiol 2006; Rosman J, et al. Lack of correlation between coronary artery calcium and myocardial perfusion imaging. 13: 333-337
(75) AJR 2006; Nikolaou K, et al. Accuracy of 64-MDCT in the diagnosis of ischemic heart disease. 187: 111-117
(76) AJR 2006; Pannu HK, et al. Coronary CT angiography with MDCT: assessment of vessel visibility. 187: 119-126
(77) J Nucl Med 2006; Berman DS, et al. Roles of nuclear cardiology, cardiac computed tomography, and cardiac magnetic resonance: noninvasive risk stratification and a conceptual framework for the selection of noninvasive imaging tests in patients with known or suspected coronary artery disease. 47: 1107-1118
(78) Radiographics 2006; Hoffman U, et al. Cardiac CT in emergency department patients with acute chest pain. 26: 963-980
(79) J Nucl Cardiol 2006; Thomas GS. Coronary computed tomographic angiography: competitive or complementary? 13: 605-608
(80) J Nucl Cardiol 2006; Wackers FJT. Asymptomatic patients with diabetes mellitus should be screened for coronary artery disease. 13: 609-615
(81) AJR 2006; Hoffmann U, et al. MDCT in early triage of patients with acute chest pain. 187: 1240-1247
(82) Radiology 2006; Leschka S, et al. Noninvasive coronary angiography with 64-section CT: effect of average heart rate and heart rate variability on image quality. 241: 378-385
(83) J Nucl Med 2006; Schuijf JD, et al. A comparative regional analysis of coronary atherosclerosis and calcium score on multislice CT versus myocardial perfusion on SPECT. 47: 1749-1755
(84) J Nucl Cardiol 2006; Wyrick JJ, Wei K. Cardiac imaging in the evaluation of patients presenting to the emergency department with chest pain. 13: 749-755
(85) AJR 2007; Muhlenbruch G, et al. Evaluation of aortocoronary bypass stents with cardiac MDCT compared with conventional catheter angiography. 188: 361-369
(86) J Nucl Cardiol 2007; Pundziute G, et al. Impact of coronary calcium score on diagnostic accuracy of multislice computed tomography coronary angiography for detection of coronary artery disease. 14: 36-43
(87) Radiology 2007; Oncel D, et al. Coronary stent patency and in-stent restenosis: determination with 64-section multidetector CT coronary angiography- initial experience. 242: 403-409
(88) AJR 2007; Schlosser T, et al. Coronary artery calcium scoring: influence of reconstruction interval and reconstruction increment using 64-MDCT. 188: 1063-1068
(89) J Nucl Cardiol 2007; Cury RC, et al. Comprehensive cardiac CT study: evaluation of coronary arteries, left ventricular function, and myocardial perfusion- is it possible? 14: 229-243
(90) J Nucl Med 2007; Gaemperli O, et al. Cardiac image fusion from stand-alone SPECT and CT: Clinical experience. 48: 696-703
(91) J Nucl Med 2007; Di Carli MF, et al. Clinical myocardial perfusion PET/CT. 48: 783-793
(92) Radiology 2007; Schoepf UJ, et al. Coronary CT angiography. 244: 48-63
(93) Radiology 2007; Herzog C, et al. Significant coronary artery stenosis: comparison on per-patient and per-vessel or per-segment basis at 64-section CT angiography. 244: 112-120
(94) J Nucl Cardiol 2007; Beller GA. CT angiography: too much too soon. 14: 267-268
(95) J Nucl Cardiol 2007; Ho J, et al. Severe coronary artery calcifications are associated with ischemia in patients undergoing medical therapy. 14: 341-346
(96) Radiology 2007; Vanhoenacker PK, et al. Diagnostic performance of multidetector CT angiography for assessment of coronary artery disease: meta-analysis. 244: 419-428
(97) AJR 2007; Matt D, et al. Dual-source CT coronary angiography: image quality, mean heart rate, and heart rate variability. 189: 567-573
(98) AJR 2007; Feuchter GM, et al. Diagnostic performance of 64-slice computed tomography in evaluation of coronary artery bypass grafts. 189: 574-580
(99) AJR 2007; Kroft LJM, et al. Artifacts in ECG-synchronized MDCT coronary angiography. 581-591
(100) J Nucl Med 2007; Schepis T, et al. Added value of coronary artery calcium score as an adjunct to gated SPECT for the evaluation of coronary artery disease in an intermediate-risk population. 48: 1424-1430
(101) Radiographics 2007; Mahesh M, Cody DD. Physics of cardiac imaging with multiple-row detector CT. 27: 1495-1509
(102) Radiographics 2007; O'Brien JP, et al Anatomy of the heart at multidetector CT: what the radiologist needs to know. 27: 1569-1582
(102) J Nucl Cardiol 2007; Schuijf JD, et al. The current status of multislice computed tomography in the diagnosis and prognosis of coronary artery disease. 14: 604-612
(103) Radiology 2007; Schuijf JD, et al. Evaluation of patients with previous coronary stent implantation with 64-section CT. 245: 416-423
(104) Radiology 2007; Das KM, et al. Contrast-enhanced 64-section coronary mutlidetector CT angiography versus conventional coronary angiography for stent assessment. 245: 424-432
(105) AJR 2007; Leontiev O, Dubinsky TJ. CT-based calcium scoring to screen for coronary artery disease: why aren't we there yet? 189: 1061-1063
(106) J Nucl Cardiol 2007; Rozanski A, et al. Use of coronary calcium scanning for predicting inducible myocardial ischemia: influence of patients clinical presentation. 14: 669-79
(107) Radiographics 2006; Pugliese F, et al. Multidetector CT for visualization of coronary stents. 26: 887-904
(108) Radiographics 2007; O'Brien JPO, et al. Anatomy of the heart at multidetector CT: what the radiologist needs to know. 27: 1569-1582
(109) Radiology 2007; Oncel D, et al. Effectiveness of dual-source CT coronary angiography for evaluation of coronary artery disease in patients with atrial fibrillation: initial experience. 245: 703-711
(110) Radiology 2007; Harmon M, et al. Coronary arteries: diagnostic performance of 16- versus 64-section spiral CT compared with invasive coronary angiography- meta-analysis. 245: 720-731
(111) Radiology 2007; Hurwitz LM, et al. Radiation dose from contemporary cardiothoracic multidetector CT protocols with an anthropomorphic female phantom: implications for cancer induction. 245: 742-750
(112) J Nucl Cardiol 2007; Di Carli MF, et al. Relationship between CT coronary angiography and stress perfusion imaging in patients with suspected ischemic heart disease assessed by integrated PET-CT imaging. 14: 799-809
(113) AJR 2007; Seifarth H, et al. Optimal systolic and diastolic reconstruction windows for coronary CT angiography using dual-source CT. 189: 1317-1323
(114) AJR 2007; Liu X, et al. Comparison of 3D free-breathing coronary MR angiography and 64-MDCT angiography for detection of coronary stenosis in patients with high calcium scores. 189: 1326-1332
(115) AJR 2007; Duerinckx AJ. Can the targeted use of MR angiography after CT angiography help assess the severity of focal calcific coronary lesions? 189: 1333-1334
(116) AJR 2008; Decramer I, et al. Effects of sublingula nitroglycerin on coronary lumen diameter and number of visualized septal branches on 64-MDCT angiography. 190: 219-225
(117) Radiology 2008; Kim DJ, et al. Saline flush effect for enhancement of aorta and coronary arteries at multidetector CT coromary angiography. 246: 110-115
(118) AJR 2008; Horiguchi J, et al. Radiation dose, image quality, stenosis measurement, and CT densitometry using ECG-triggered coronary 64-MDCT angiography" a phantom study. 190: 315-320
(119) J Nucl Cardiol 2008; Yerramasu A, et al. Cardiac computed tomography and myocardial perfusion imaging for risk stratification in asymptomatic diabetic patients: a critical review. 15: 13-22
(120) J Nucl Cardiol 2008; Di Carli MF, Hachamovitch R. Hybrid PET/CT is greater than the sum of its parts. 15: 118-122