PET Myocardial Imaging:
Presently SPECT imaging is responsible for the great majority of cardiac imaging applications. Findings on the SPECT exam have both diagnostic and prognostic value. PET imaging has certain advantages including higher spatial resolution, improved attenuation correction, and the capability to perform quantitative measurements [17,37,46]. Like SPECT, PET myocardial imaging can also provide diagnostic and prognostic information. In the detection of coronary artery disease, PET imaging has a sensitivity of 92%, a specificity of 89% (higher than SPECT--75%), and a normalcy of 89 to 95%. The higher specificity is related to the capability of PET imaging to provide attenuation correction that decreases the false positive rate. However, reconstruction artifacts producing false-positive defects can occur in up to 21% of cases due to mis-registration between transmission and emission scans [32]. Careful review of superimposed transmission and emission data sets should be performed to ensure correct registration [32]. Acquiring separate rest and stress transmission scans can help to reduce mis-registration artifacts [32].
At the present time, stress testing with PET must be performed with a pharmacologic agent [29]. Because the exercise component of a stress test in patients who are able to exercise provides independent prognostic information, the use of PET imaging should be reserved for patients unable to exercise, those with a prior equivocal SPECT exam, or in whom body habitus would degrade SPECT imaging [29].
PET Perfusion Imaging
Physiology of perfusion imaging
PET perfusion imaging
Another advantage of PET perfusion imaging compared to SPECT imaging is that global and regional myocardial blood flow can be quantified in ml/gm/min. The benefits of quantification are that the functional significance of coronary stenoses can be assessed directly, multivessel disease producing balanced ischemia can be detected, and the effects of medications or interventional cardiac procedures can be objectively measured.
The ideal PET perfusion agent would be irreversibly trapped in the myocardium in direct relation to perfusion and have rapid blood clearance. The agents most commonly used for myocardial blood flow determinations are 13N-ammonia (13NH3), 15O labeled water (H215O), and 82Rubidium [29]. Because of poor extraction at high flow rates and relatively worse spatial resolution, 82Rubidium provides the least accurate estimate of myocardial blood flow [33]. 15O labeled water is theoretically superior to 13N-ammonia because it is a freely diffusible tracer with virtually complete myocardial extraction that is nearly independent of both flow rate and myocardial metabolic state [33]. When 15O labeled water is used, the myocardial blood flow (MBF) is estimated from the tracers washout from the myocardium, whereas for 13N-ammonia the myocardial tracer uptake is used to determine MBF [33].
The initial minutes of a dynamic
acquisition are the portion of the exam that is used for blood flow
quantification. Because of the limited count rate capabilities of PET scanners
and varying tracer kinetics, mathematic models must be applied for proper
quantification [29]. Methods have been developed to separate the delivery of tracer
to the heart (blood activity) from tracer retention in the myocardium.
Myocardial
activity is determined by a region of interest (ROI) placed over the myocardial
wall. Blood activity can be measured accurately by direct sampling of blood from
an artery, but this is invasive and the sample would still require correction
for time delays and dispersion. Measurements performed from the reconstructed
PET images would be more practical and less invasive.
The
most accurate site to measure arterial blood pool activity is at the level of
the coronary ostia at the base of the ascending aorta because this is the point
from which arterial blood irrigates the myocardial tissue. Unfortunately,
measuring activity from a ROI placed within the ascending aorta is subject to
error due to the limited resolution of the imaging system, the small dimensions
of the aorta, and contamination from adjacent vascular and myocardial
structures. Thus, activity is usually measured from an ROI placed within the
left atria or left ventricle. The drawback of a left ventricular ROI is that it
is subject to motion artifact as well as spillover activity from myocardial
tissue. A left atrial ROI tends to closely match values obtained from direct
arterial sampling, however, it is often more difficult to identify the left
atrial chamber on PET images. When using a left atrial ROI it is important to
correct for the small time delay between the arrival of the blood in the left
atria and its actual arrival in the myocardium or flow rates may be
artificially elevated.
PET/CT and attenuation correction:
Previously, attenuation correction of myocardial imaging was performed with a 68Ge transmission source [31]. The typical radiation dose from a 68Ge transmission scan was about 8 mrem (0.08 mSv) [45]. Newer PET/CT scanners use CT for attenuation correction and this has also been shown to provide accurate results for qualification and quantification of myocardial perfusion [31]. A low dose CT used only for attenuation correction results in a radiation dose of about 80 mrem (0.8 mSv) [45]. Following completion of the PET study, a coronary CT angiogram can be performed [52]. By coupling the perfusion exam findings to a coronary CTA study, PET/multi-detector CT permits the fusion of anatomic and functional information [37,39]. The fused exams can accurately measure the atherosclerotic burden and identify the functional significance of coronary stenoses [37,39]. The results of the combined exams can more accurately identify patients for revascularization [39]. CTA imaging should be performed following the PET study to avoid potential interference from beta-blockers used for heart rate control [52]. The radiation dose to the patient from combined coronary CTA and PET rest-stress myocardial perfusion imaging is between 10-12 mSv [39].
Both rest and stress exams should have separate CT transmission scans for attenuation correction [52]. For stress images, the best attenuation correction results are usually obtained with post-emission scanning (i.e.: following completion of the PET stress exam) [52]. The effects of the vasodilator stress have usually passed by this time and patients are more comfortable and less likely to move [52].
When using CT for attenuation correction of PET images, certain limitations exist.
1- Respiratory motion: Respiration causes the heart to move up by as much as 1.6 cm- which is about the thickness of the heart wall and this will blur the PET image [54]. Previously, transmission data sets were acquired using a germanium source. As with the emission data set, the transmission scan was acquired over several minutes and the two blurred data sets would then be closely matched (if the patient did not move between acquisitions) [53]. With CT for attenuation correction, the CT exam freezes respiration at a single point in the respiratory cycle, while the PET emission data is still acquired over several minutes [48]. If the PET and CT data sets are not properly aligned, respiratory effects can introduce large inhomogeneities in apparent myocardial uptake (up to 30% decreased or increased activity compared to actual uptake) [48,53]. This is particularly problematic for the lateral and anterior walls of the left ventricle as the myocardium has a boundary with the lung and a few millimeters of respiratory motion can easily make the myocardium in the emission data appear in the lung field of the CT data resulting in improper attenuation correction (the myocardium in the portion of the lung will be undercorrected due to differences in attenuation factors between the heart and lung) [48,49,51,54,56]. Misalignment of more than 6 mm can produce noticeable effects, but the greater the degree of misalignment, the more conspicuous the defects [49,56]. Misregistration of more than 6 mm can result in artifactual PET defects in up to 40% of patients, and these defects are moderate to severe in 23% [56]. In up to 18% of patients, perfusion defects can completely disappear following reprocessing with manual registration of the PET and CT data sets [49]. The septal and inferior walls are in contact with other soft tissue structures so that a misalignment will produce only minor changes in the attenuation factors for these segments [49].
Various methods have been proposed to correct the problem of mis-registration between the PET and CT data sets-
A. End-expiration CT imaging: Mis-registration and variation have been shown to be minimized when CT images are acquired at end-expiration [48].
B. Respiration-averaged CT or ultraslow CT acquisition [49,51,54]. Have been shown to be superior to end-expiration CT.
C. Gating- The most eloquent way to correct for respiratory motion is to gate the CT to the respiratory cycle and then average the respiratory gated data to match the ungated PET data [51]. Alternatively, the both the PET emission scan and CT could be acquired with respiratory gating- the attenuation corrected data at each phase of the respiratory cycle would be matched to the emission data from the corresponding phase [51]. However, this type of phase matched acquisition would require a large amount of data processing and would be difficult to implement clinically [51].
D. Cine CT- A cine CT exam is acquired during the duration of a respiratory cycle and is used for attenuation correction. However, up to 19% of patients may still require manual registration of the PET and cine CT data set to ensure proper attenuation correction [56]. The intensity-maximum cine image seems to be the best for improving attenuation correction and has been shown to be superior to end-expiration CT. [53]. The cine CT exam exposes the patients to additional radiation (about 2 mSv when acquired using 10 mA and 140 kVp for approximately a 6 sec acquisition) [53].
2- Metallic artifacts: Metal associated with cardiac pacers and implantable defibrillator devices can produce image artifacts on cardiac PET/CT imaging [38]. Most metals exhibit strong photoelectric absorption of x-rays, but interact with 511 keV gamma rays primarily via Compton scattering [38]. The CT attenuation correction scaling algorithm does not account for this effect and causes overcorrection of the PET images (i.e.: it produces a "hot" spot) [38]. Overcorrection is not a signifnicant problem with conventional PET scanners that use 68Ge/68Ga or 137Cs transmission sources with gamma energies of 511 keV and 662 keV, respectively [38]. Defibrillator leads can be particularly problematic if they are placed in close relationship to the left ventricle (typically within 15 mm) [38]. Falsely elevated FDG uptake of 44-81% be seen at the lead location [38]. This focal area of increased activity can mask a perfusion defect and can also interfere with image normalization (i.e.: the remainder of the myocardial segments can appear to have less tracer uptake) [38]. Pacemakers produce a more moderate focal abnormality [29,38]. A segmented reconstruction algorithm for the CT scan may help to overcome artifacts from metallic implants [52].
Radiopharmaceuticals/Technique for perfusion imaging
Agents used for PET myocardial perfusion imaging include 13N-ammonia, 15O-water, Rubidium-82, and 62Cu -PTSM. Agents labeled with 18F (such as 18F-fluorodihydrorotenone) are being developed for clinical use [36].
13N is cyclotron produced by either irradiating methane gas with deuterons [12C (d,n) 13N] or by the reaction 16O (p,alpha) 13N with water as the target. 13N has a physical half-life of 10 minutes. Its positron range is only 0.4 mm which results in excellent images [44]. There is rapid blood clearance with high initial extraction (over 90%) and high tissue retention (80%), which provides high myocardial to background count ratios. The agent readily diffuses across plasma and cell membranes [52], although the exact mechanism of intracellular transport remains unclear [44]. Tracer uptake in the lungs is usually minimal, but it can be increased in smokers and in patients with congestive heart failure and can interfere with imaging [29,52]. In these patients, it may be necessary to increase the time between injection and imaging to improve myocardial to background activity [52]. Liver uptake can also be problematic [29,52].
Inside the myocyte the agent is metabolized to 13N-labeled glutamine via glutamine synthetase and becomes unable to leave the cell (only a small fraction diffuses back into the intravascular space) [44,52]. Extraction of the tracer generally reflects regional perfusion, however, extraction is non-linear and decreases at high flow states (to as low as 35%) and plateaus at flow rates greater than 2 mL/min/gm [29]. This leads to underestimation of flow at high flow rates [52]. According to Di Carli- the first pass extraction of the agent is 83% when blood flow is 1mL/min/g and decreases to 69% at a flow of 3mL/min/g [52]. Di Carli also states that extraction is linear for flow rates below 2.5 mL/min/g [52]. At the higher flow rates "metabolic trapping" of the agent becomes the rate limiting factor affecting tracer retention [52]. Another consistent finding is low uptake of the tracer in the lateral/posterolateral wall (about 10% reduction even in healthy subjects) on both stress and rest images which appears to be related to a regional alteration in 13N tissue metabolism/retention [3,40,52]. This defect is not detected on dynamic imaging, which can be used to differentiate a true perfusion defect from artifact [4].
The critical organ for N-13 is the urinary bladder. The typical dose used for imaging is 10 to 15 mCi I.V. given over a 20 to 30-second interval. Di Carli recommends using a lower activity for the rest exam (about 10 mCi) and a higher activity for the stress study (about 30 mCi)- this avoids having to wait for the rest dose to decay to background levels prior to performing the stress exam [52]. Imaging is usually started 3-5 minutes after injection of the tracer to permit pulmonary background activity to clear and the scan duration is about 20 minutes [52]. Images should be acquired in a decay compensated mode. Because the agent has a 10 minute half-life, post-treadmill exercise is feasible [44]. For a typical rest-stress 13N exam the effective radiation dose is about 2.2 mSv [43]. Images can be gated to provide assessment of regional and global cardiac function [52]. A FDG exam may be performed after a 50-minute interval to permit decay of the N-13 to background levels. Quantifcation of blood flow can be performed with 13N-ammonia and has been well validated [52].
15O is cyclotron produced by the irradiation of nitrogen [14N (d,n) 15O or 15N(p,n)15O] and it has a physical half-life of only 2.2 minutes (123 seconds). O-15 produced in the cyclotron is converted to [15O]CO2 by passing it over activated charcoal at 400 to 600 degrees Celsius. [O-15] water is then prepared by bubbling the [15O]CO2 into water. Theoretically, 15O water is ideal for quantitative regional myocardial flow measurements (in ml/min/gm) for two reasons: it is a freely diffusible perfusion tracer with 100% extraction by the myocardium [29], and it is not affected by metabolic factors. The positron range is about 1.1 mm [44].
The extraction of oxygen-15-water (H215O) remains linear even at very high flow rates (it is the only perfusion tracer with linear extraction- meaning its accumulation in tissue is almost exclusively a function of blood flow [44]) and, therefore, myocardial distribution of the agent reflects regional perfusion. Unfortunately, image quality is not as good as that obtained with other flow agents because tracer circulating in the blood pool remains within the ventricular chamber and must be subtracted in order to visualize the myocardium. This can be done by use of a separate administration of O-15 carbon monoxide to label erythrocytes or by the use of an early image (20-40 seconds after the administration of O-15 water) which would represent a vascular image before significant activity reaches the coronary arteries [29].
For myocardial perfusion quantification, dynamic scans are obtained for up to 5 minutes after bolus administration of 15-25 mCi [29]. For a typical rest-stress H215O exam (2 x 740 MBq) the patient effective radiation dose is about 1.4 mSv [43].
Rubidium-82 (82Rb) is a generator produced positron emitter. Rubidium-82 has a physical half-life of only 75 seconds. The agent is produced from a Strontium-82 generator (T1/2=25.5 days) which decays by electron capture to 82Rb. The generator can therefore be used for about 1 month [44]. The generator can be eluted with a greater than 90% yield every 10 minutes [52]. Rubidium-82 is a potassium analog and tissue uptake requires an active Na/K-ATPase pump for intracellular transport [44]. The short half-life allows for repeat imaging and blood flow measurements in short time intervals, but requires the use of large doses of the tracer. However, the short half-life of the agent has some negative effects as well. First- it is crucial to wait for 82Rb to clear from the blood pool prior to imaging because 82Rb counts in the cavity could scatter into subendocardial defects and obscure them [43]. The time required for clearing of 82Rb from the cardiac blood pool is inversely related to the cardiac output and directly related to the circulation time [43]. Therefore, following injection imaging must be delayed for at least 2 minutes to permit clearance of blood pool activity and considerable decay of the tracer occurs during this time. Also, due to changing myocardial activity from decay, imaging times must be short to prevent back-projection reconstruction artifacts. Another drawback of the agent is that myocardial single-capillary transit extraction of the agent is low- about 50-60% at rest [52] and this further decreases at high flow rates to only 25-30%. However, another article suggests high extraction of 82Rb by the myocardium and that uptake is more linearly related to increases in coronary blood flow than Tc-99m SPECT agents [46]. Myocardial ischemia and reperfusion reduce the uptake of Rb-82 due to a reduction in cellular transport [29]. This reduction in extraction occurs even after only short periods of transient ischemia [29]. 82Rb has the worst resolution of all the positron emitting agents- this is because of it's high positron energy (1.52 MeV) which results in a mean range of about 5.5 mm (2.8-12.4 mm) prior to undergoing an annihilation [44,46].
Radiation dose: The critical organ is the kidney. For a typical rest-stress exam the effective patient dose is about 302-500 mrem (5.0 mSv) [43,46] to 1.6 rem (16 mSv) [45]. The dose is lower or similar to that associated with rest-stress Tc-99m perfusion imaging (approximately 667 mrad) [46].
|
Rb-82 rest perfusion examination |
|
|
Exam protocol: Rest imaging should be performed prior to stress imaging to reduce the impact of residual stress effects such as myocardial stunning which can affect Rb-82 extraction [27,29]. Pharmacologic stress using dipyridamole is presently used for 82Rb imaging [42,46]. The longer duration of hyperemia with dipyridamole enables acquisition of three separate image sets- transmission, emission, and ECG-gated [46]. Following the exam, aminophylline 100 mg can be administered to reverse the effects of dipyridamole [46].
On SPECT pharmacologic imaging, ST segment depression during vasodilator infusion has been shown to be associated with an increased risk for subsequent cardiac events even when perfusion imaging was normal [42]. However, a normal 82Rb stress exam confers an excellent prognosis even when ST segment depression is noted [42].
The typical dose for the 82Rb perfusion exam is 40 to 60 mCi (1.48-2.22 GBq) infused over 20 to 30 seconds [27,46,52]. In patients with normal LV function, imaging is usually begun 60-70 seconds (Di Carli recommends 90 sec in healthy individuals) after the infusion is complete to permit clearance of blood pool activity. Imaging is delayed to 90-120 seconds following completion of the infusion in patients with poor LV function (LVEF less than 30%) or RV systolic function [27,52]. About 80% of the useful counts are acquired in the first 3 minutes, and 95% in the first 5 minutes [27].
For a standard 2D acquisition 82Rb cardiac images tend to be count poor due to the agents short half-life [26]. 3D imaging has a higher sensitivity, however, it suffers from greater scatter, random events, and saturation which can paralyze a system [26]. Further work needs to be performed to determine optimal 3D myocardial perfusion imaging [26].
Results: Compared to SPECT imaging, PET imaging with Rb-82 has been shown to be more accurate (87% versus 71% for greater than 50% vessel stenosis), to have fewer artifacts (due to lower bowel and liver activity which affected only 5% of PET studies, but 41% of SPECT exams), and to produce overall higher quality examinations which results in a higher diagnostic confidence (i.e.: a greater number of definitively normal or abnormal exams) [46]. PET imaging was also superior for localizing disease to individual coronary arteries [46]. Identification of multivessel CAD is also superior with PET imaging [46]. As with SPECT imaging, the extent and severity of stress perfusion defects correlate with an increased risk for mortality [52]. Preliminary data indicate that a normal PET exam is associated with a very good prognosis and a low cardiac event rate (0.4% rate of MI or cardiac death) [52]. In one study, the use of PET perfusion imaging in patients with an intermediate probability for coronary artery disease was found to reduce the use of invasive coronary angiography by over 50% (a 30% cost savings) [55]. This was felt to be related to the improved specificity of PET compared to SPECT imaging (false positive exams decreased from 15.6% with SPECT to 5.2% with PET) [55].
However, a normal stress perfusion PET study is an overall poor discriminator of patients without non-flow limiting (subclinical) coronary atherosclerosis [60]. Up to 50% of patients with normal PET perfusion imaging are found to have some degree of CAD by CTA [60]. Therefore, CTA imaging plays a complimentary role to PET perfusion imaging by permitting identification of pre-clinical CAD that can be treated with aggressive medical treatment and life style modification [60].
Quantitative evaluation: Quantification of blood flow can be performed with Rb-82, but is challenging [52]. Because true determination of absolute perfusion is complex and time consuming, balanced 3-vessel ischemia may still not be readily apparent even in perfusion PET imaging. Unlike SPECT imaging which is performed in a delayed manner, 82Rb PET enables measurement of LV function during peak pharmacologic hyperemia [50]. Even with pharmacologic stress, LVEF increases during vasodilator stress in normal patients [52]. A reduced subendocardial flow reserve or a coronary steal in patients with multivessel disease can lead to myocardial dysfunction with pharmacologic stress and changes in the LVEF [50]. A decrease in LVEF of more than 5% during pharmacologic stress can be seen in patients with severe multivessel CAD [50]. Conversely, an increase in LVEF of more than 5% has a very high negative predictive value for excluding the presence of severe left main or 3-vessel CAD (i.e.: the negative predictive value of a greater than 5% increase in LVEF between rest and stress to exclude the presence of 3-vessel or left-main disease is 97%) [50]. LVEF has been shown to provide additional prognostic information over stress perfusion PET imaging alone [52].
Copper 62 PTSM: Copper-62-Pyruvaldehyde-bis-(4N-thiosemicarbazone)
Cu-PTSM is a generator-produced tracer which can also be used for myocardial perfusion imaging. 62Cu has a physical half-life of 9.7 minutes. The short half-life of the parent isotope 62Zn (9.3 hours) limits the practical life of the generator to 1 to 2 days. However, once loaded, the generator can be eluted every 30 minutes. The positron range is 2.7 mm [44].
62Cu -PTSM is a neutral, lipophilic tracer that is taken up in tissues and then trapped intracellularly by reduction to a non-lipophilic compound. There is non-linear extraction of the tracer (ie: extraction decreases at high flow rates) and high liver activity which scatters into the inferior wall. The agent also binds to human albumin which precludes accurate recording of the arterial input function which is critical for quantification. [5] The recommended dose is 0.12-0.2 mCi/kg as an intravenous bolus followed by dynamic imaging for 10-15 minutes [44].
PET Metabolic Imaging
There are numerous clinical applications for PET metabolic myocardial imaging. One of the most recognized applications is for the assessment of viable myocardial tissue. As with perfusion, myocardial metabolic functions can also be quantified by PET imaging.
Physiology of metabolic myocardial imaging:
The heart can oxidatively metabolize a variety of substrates to meet its high energy needs. Under post-prandial conditions, plasma insulin levels rise, peripheral lipolysis is inhibited, and there is increased myocardial glucose metabolism [41]. In the fasting state (low insulin levels, increased lipolysis in peripheral adipose tissue, and high circulating free fatty acids [41]) the myocardium preferentially utilizes fatty acids and lactate as aerobic substrates to provide the energy (ATP) necessary for contractile function (unlike other cells in which glucose is the most important substrate). In plasma, fatty acids are transported bound to albumin and enter the myocardial cell either by passive or active transport. Once in the cell, fatty acids undergo oxidative metabolism. Although fatty acids are the preferred myocardial energy substrate, even in the fasting state glucose utilization is variable and can still account for 30-40% of the energy derived from oxidative metabolism [15]. Addtionally, there is an age-related decrease in fatty acid metabolism and a relative increase in glucose metabolism in healthy older individuals [41].
Fatty acid beta-oxidation occurs in the mitochondria and is very sensitive to oxygen deprivation. When blood flow is significantly reduced, tissue delivery of oxygen and removal of waste products are also reduced. As a result, oxygen dependent substrate catabolism decreases. Since fatty acids are catabolized by beta oxidation, fatty acid consumption ceases with anoxia (i.e.: a depression of fatty-acid extraction and oxidation will occur in zones of myocardial ischemia or infarction). This decrease in fatty acid utilization is followed by an increased glycolytic flux with glycogen depletion and increased exogenous glucose utilization.
During periods of ischemia or hypoxia the myocyte compensates for the loss of oxidative potential by shifting toward glucose utilization to generate high-energy phosphates [41]. Unfortunately, the amount of energy produced via glycolysis may not be sufficient to sustain mechanical work, but it can be adequate to maintain cell viability--this condition is referred to as hibernating myocardium. Glycolysis can only be maintained if lactate and hydrogen ions (the byproducts of glycolysis) do not accumulate intracellularly. Therefore, blood flow must be sufficient to deliver glucose to the cell and remove the metabolites of the glycolytic pathway. Once perfusion is decreased below a critical level, the tissue concentrations of lactate and hydrogen ions will increase and inhibit glycolysis [14]. This results in a loss of ion concentration gradients across the cell membrane, followed by cell membrane disruption, and cell death [14].
PET Metabolic Myocardial Imaging using 18F-2-Deoxyglucose (18-FDG) and exam technique:
18F-2-deoxyglucose is one of the agents used for cardiac metabolic imaging. Fluorine-18 has an effective half life of 110 minutes. It is produced by bombarding 18O with protons and displacing a neutron [18O (p,n) 18F]. Chemically 18F is comparable in size to hydrogen. 18F has the best resolution of all positron emitters--approaching 2 mm. This is because most of the emitted positrons traveling only about 1.2 mm prior to undergoing an annihilation reaction. For a 10 mCi 18FDG injection, the effective patient dose is about 7 mSv [43,45].
The agent 18FDG competes with glucose for facilitated transport into myocardial cells and once inside the cell it competes for phosphorylation by hexokinase. Unlike glucose, however, the phosphorylated form is not further metabolized. Therefore, regional myocardial uptake of 18FDG reflects regional rates of exogenous glucose utilization. Only about 1 to 4% of the injected dose is trapped in the myocardium, but the target to background ratios are favorable (Heart:Lung [20:1], Heart:Blood [14:1]).
|
.Myocardial PET FDG
imaging: |
|
|
18FDG uptake in the myocardium is highly dependent on the patient's dietary state [14]. Myocardial glucose utilization is increased by glucose administration (ie: such as following a meal) which stimulates insulin secretion. The increased insulin levels stimulate glucose metabolism, and tissue lipolysis is inhibited [15]. Therefore, in normal myocardium, 18FDG, uptake will be promoted in association with high glucose/insulin levels, but decreased in the face of high free-fatty acid levels (ie: with fasting). Unlike normal myocardium, regions of severely decreased perfusion (hibernating myocardium) preferentially utilize glucose as an energy substrate during periods of fasting in order to maintain cell viability. It is this difference in substrate utilization between normal and hibernating myocardium that forms the basis for PET myocardial viability imaging.
For metabolic imaging, patients fast for at least 6 hours and then receive a glucose load [27]. Several protocols are available to promote cardiac FDG uptake:
- Oral glucose loading
- Hyperinsulinemic euglycemic clamping
- Administration of nicotinic acid derivatives
In patients with diabetes the increase in plasma insulin levels following glucose loading may be attenuated and the cells may be less able to respond to insulin stimulation [15,27]. Consequently, tissue lipolysis is not inhibited, plasma fatty acid levels remain high, and myocardial glucose uptake can be poor [15]. This can result in suboptimal quality exams. An improvement in image quality can be obtained by waiting 2-3 hours after tracer injection before imaging (at the expense of increased FDG decay) [27]. In diabetic patients following glucose loading, an IV bolus of regular insulin is given according to a sliding scale and the plasma glucose is checked every 15 minutes in order to maintain a stable serum glucose level of approximately 140 mg/dL.
FDG imaging in patients with non-insulin dependent diabetes is also problematic [20]. An oral glucose load and insulin boluses can be used to control blood glucose levels [20]. For a fasting plasma glucose level less than 7 mmol/L, a 25 g oral glucose solution is given; for 7-11 mmol/L , 5 IU of insulin is given I.V.; for a fasting glucose greater than 11 mmol/L, 10 IU of insulin is given I.V. [20]. The plasma glucose level is then measured in 15 minutes and insulin given according to a sliding scale: For 8 mmol/L no insulin is given; for 8-11 mmol/L, 5 IU of insulin is given I.V., and for a glucose level greater than 11 mmol/L, 10 IU of insulin is given I.V. [20].
Hyperinsulinemic-euglycemic clamping: Hyperinsulinemic-euglycemic clamping requires the simultaneous infusion of glucose and insulin to achieve perfect metabolic regulation [22]. With the use of the clamp, high insulin levels, stable glucose levels, and low free fatty acid levels can be obtained [22]. The hyperinsulinemic-euglycemic clamp method has been shown to produce images with the highest myocardial to background ratio [20] even in diabetic patients, however, the method is time consuming and not practical for clinical work [15]. The procedure requires IV's placed in both the left and right arms- one is used for insulin infusion and the other for glucose. Insulin is infused at a rate of 100mU/kg/hr to achieve hyperinsulinemia [22]. A simultaneous glucose infusion (500mL of 20% glucose with 20mL of 14.9% potassium chloride to prevent hypokalemia) is infused at a rate ot 6 mg/kg/min and the rate is adjusted every 10 minutes to maintain normoglycemia [22]. After 60 minutes of clamping, the FDG is administered intravenously [22].
Nicotinic acid derivatives: Nicotinic acid derivatives have also been studied for use with PET FDG myocardial imaging [22]. The agent acipimox inhibits peripheral lipolysis and thereby reduces circulating free fatty acids [22]. Acipimox 250 mg is orally given 2 hours prior to FDG injection. Some centers will also give patients a carbohydrate and protein-enriched meal [22]. Image quality is generally very good (even in patients with diabetes [25]), with uninterpretable exams in about 5% of patients [22]. The effectiveness of this agent in patients with diabetes requires further study [22].
The typical dose used for the exam is 15 mCi infused over a 60 second interval. Images are usually acquired 45 minutes following administration of the radiotracer [30]. When performing attenuation correction with the use of a transmission scan, ideally the transmission scan should be performed prior to FDG injection. Post-injection transmission scans may suffer from emission contamination and the resultant attenuation corrected images can underestimation FDG activity by about 20% unless an emission spillover adjustment is applied [30].
PET FDG imaging for the detection of viable myocardial tissue:
See also discussion for Viability imaging in the cardiac section
Hibernating myocardium represents severely ischemic, but viable myocardial tissue. In this condition there is a chronic reduction in myocardial metabolism and contractility in order to match a long-standing decrease in blood supply (chronic ischemia). In other words, the cells maintain viability, but energy production is insufficient to maintain mechanical work and contractile dysfunction results [1]. This may be a protective response by the myocytes to reduce their oxygen demand in the setting of reduced oxygen availability. This is a reversible phenomenon and ventricular contractile dysfunction can improve if flow is restored. Because enhanced left ventricular function after revascularization is associated with improved survival, it is crucial to identify areas of viable myocardial tissue.
Evaluation of glucose metabolism using 18FDG remains the gold standard for the evaluation of myocardial viability (hibernating myocardium) [1,6,19]. Ischemic, but viable myocardium (hibernating) utilizes glucose in preference to other substrates. Ischemic myocardium will therefore concentrate an increased amount of 18FDG compared to normal myocardium. Increased utilization of glucose can also be seen in myocardium recovering from a recent ischemic event as enhanced anaerobic glycolytic activity persists for some time after reperfusion, despite the availability of adequate oxygen.
The viability examination is usually performed following some form of glucose loading even though this seems to be contradictory. It would seem most logical to perform 18FDG myocardial viability imaging in the fasting state when normal myocardial glucose utilization is very low (normal myocardium uses fatty acids for energy substrate in the fasting state). Unfortunately, fasting 18FDG imaging of the myocardium may be overly sensitive -- detecting small, clinically insignificant areas of myocardial viability because of low background myocardial activity. Additionally, the normal myocardium can be difficult to define in the fasting state and the tracer may not clear adequately from the blood, which can result in image degradation. The one drawback of glucose loading exams is that myocardial substrate utilization will shift from fatty acids to glucose and small areas of viable myocardium may be missed. In either case, the average positive predictive accuracy for detecting viable myocardium by fasting FDG studies is about 85% and is similar to post-glucose loading exams (81%).
Severely decreased FDG uptake below 50% of normal myocardial activity is generally considered indicative of scar, while uptake greater than 50% of normal myocardial activity is indicative of viability. However, determination of ischemic, but viable myocardium can only be made in relationship to a perfusion study [7]. The perfusion exam does not need to be performed with a PET agent; it can be performed using SPECT tracers such as thallium or technetium agents [18]. When regional myocardial 18FDG uptake is disproportionately enhanced when compared with regional myocardial blood flow, the pattern is termed a perfusion-metabolism mismatch. In the setting of chronic coronary artery disease, a perfusion-metabolic mismatch is highly predictive of myocardial viability and indicates a high likelihood of improved cardiac function following revascularization (improved contractility is seen in 80%-85% of cases and the average improvement in ejection fraction (EF) is 15%).
A scar is characterized by concordant reduction in perfusion and FDG uptake (a FDG-perfusion match) [14]. Improved wall motion is seen in only 8-17% of matched perfusion-metabolic defects [14]. Based on the severity of the perfusion and FDG deficit, the matched pattern may be categorized as a transmural match (absent or markedly reduced perfusion and FDG uptake) or a non-transmural match (mild to moderately reduced perfusion and FDG uptake). When tracer activity equals 50 to 60% or more of peak activity, the matches are mild and probably represent a non-transmural scar [14]. Contractile reserve is more likely to be seen in regions of non-transmural MI [23]. If quantification is performed, viable myocardium is very unlikely to exist if the basal myocardial blood flow is less than 25 ml/min/100 gm [8].
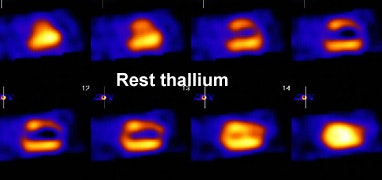
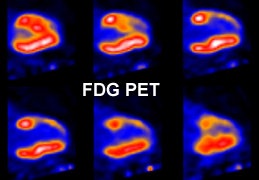
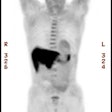
Myocardial scar: The patient below was being considered for coronary bypass surgery. A rest thallium exam demonstrated findings consistent with an apical and anterior wall scar. FDG PET imaging was requested to confirm that the patient was not a surgical candidate. The PET scan demonstrated a severe apical and anterior wall metabolic defect with less than 50% of peak myocardial activity corresponding to the thallium scan abnormality. The finding was consistent with prior apical and anterior wall infarct. |
|
Although the uptake of 18FDG can discriminate between viable and non-viable tissue, the regional myocardial blood flow and 18FDG uptake pattern is similar for varying types of myocardial dysfunction. Clinical symptoms are necessary to determine the exact etiology of the scan findings. For instance, increased utilization of glucose can also be seen in myocardium recovering from a recent ischemic event as enhanced anaerobic glycolytic activity persists for some time after reperfusion, despite the availability of adequate oxygen. Different conditions may also coexist in the same myocardial regions.
Condition |
rMBF |
FDG Uptake |
Clinical State |
| Acute ischemia | Decreased | Normal or increased | Chest pain/Angina |
| Hibernating | Decreased | Normal or increased | Chronic stable |
| Stunning | Normal | Normal or increased | Following acute event |
| Necrosis/Scar | Decreased | Decreased | Chronic symptoms |
Prognostic Implications of FDG PET Myocardial Imaging (General):
Cardiac events and mortality:
PET studies have shown that cardiac morbidity and mortality is increased in patients with flow-metabolic mismatches [18,57]. Up to 50% of patients that demonstrate a perfusion-metabolic mismatch will have a cardiac event in the subsequent 12 months in the absence of intervention. The greater the number of mis-matched segments identified, the greater the risk for subsequent cardiac event if revascularization is not performed [18]. The incidence of cardiac events drops to 15% in these patients if revascularization is performed [9]. In two other studies, mortality ranged between 4 to 12% in the group with matched defects, and between 33 and 41% in the mismatch group. In the mismatch group, if revascularization was performed, mortality dropped to between 4 and 12% [7]. In a meta-analysis of patients with viable myocardium, revascularization was found to be associated with an approximately 80% reduction in annual mortality [44].
In patients with underlying left ventricular dysfunction:
It is well recognized that in patients with coronary artery disease (CAD) on medical therapy, the presence of left ventircular (LV) dysfunction is associated with a high mortality. In the Coronary Artery Surgery Study (CASS), mortality of medically treated patients was related to the severity of LV dysfunction with up to 25% annual mortality in patients with resting LVEF's below 25%. In patients with LVEF's below 35% survival was better in those patients undergoing revascularization. Unfortunately, revascularization cannot be recommended in all patients with poor LV function because the surgery is associated with a 5 to 35% mortality in this subgroup. Ideally, the surgery should be performed only in those patients with a high likelihood of improved LV function.
In patients with symptoms of cardiac failure a PET pattern of perfusion-metabolic mismatch identifies a subgroup of patients who are at very high risk for cardiac death on medical therapy (30-40%). These patients are most likely to show significant clinical improvement and have prolonged survival as a result of revascularization [1,18]. Hence, PET can be used to select the appropriate cohort of patients most likely to benefit from surgery and avoid the cost and risks of this procedure if the patient will have no benefit (resulting in an overall cost reduction) [47]. PET results can alter clinical decision making regarding revascularization in approximately 60% of patients [47].
For the prediction of improvement in regional LV function after revascularization, 18FDG -PET imaging has a pooled sensitivity of 88-93%, a specificity of 58-73%, a positive predictive value of 76%, a negative predictive value of 86%-88% [14, 19, 21,57], and an accuracy of 74%-95% [16]. A matched perfusion-metabolism defect is 75%-100% accurate in predicting that segmental wall motion will not improve [16]. Improved wall motion is seen in only 8%-17% of matched perfusion-metabolic defects [14]. Unfortunately, not all patients with evidence of viable myocardium on PET imaging demonstrate improvement in wall motion following revascularization [24]. Despite a substantial amount of viable myocardium these patients may have non-transmural infarcts with endocardial necrosis (the endocardium contributes almost 75% to total wall thickening at rest) [24] or severe ultrastructure damage which limits contractile reserve [34].
For overall left ventricular function, as a general rule, the larger the extent of the perfusion-metabolic mismatch, the greater will be the anticipated improvement in LVEF following revascularization [34]. If 17% [37] to 25% or more of the LV is viable, a significant improvement in LVEF can be expected following revascularization [14]. Patients with large mismatches (more than 18% of the myocardium [44]) also achieve significantly higher functional status compared to patients with minimal or no PET mismatch [34,44]. On average, LVEF has been shown to improve about 10 percentage points in patients with viable myocardium [57].
The perfusion-metabolism mismatch pattern is also predictive of improvement in heart failure symptoms after revascularization [10]. In patients with heart failure symptoms and a PET perfusion-metabolic mismatch, 70% had improvement in symptoms following revascularization, while only 30% without a mismatch experienced improvement. Improvement in heart failure symptoms correlates with the preoperative extent of viable myocardium [37]. Patients with a PET mismatch pattern involving 18% or more of the LV volume appear to show the greatest clinical benefit [37]. In patients with ischemic cardiomyopathies being considered for revascularization, LVEF and LV volume information determined from gated FDG PET provide incremental prognostic value [35]. Patients with LVEF's below 25% or EDV's greater than 260 mL may not demonstrate improvement in heart failure symptoms following revascularization (although they may still derive a survival benefit) [35]. This is most likely related to advanced ventricular remodeling [35].
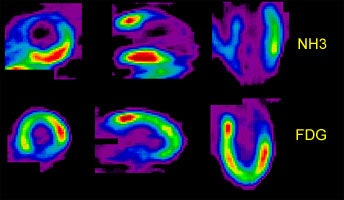
Perfusion-Metabolism Mismatch: The case below is from a 62-year-old female patient 8-years status post CABG and 6 months following an anterior wall myocardial infarction. The patient presented with symptoms of congestive heart failure and a LV ejection fraction of 22%. Perfusion exam was performed using NH3. There is an extensive, severe perfusion defect involving the apex, anterior, and septal walls. Metabolic images performed using FDG demonstrate disproportionately enhanced uptake when compared to the perfusion study. This is referred to as a perfusion-metabolism mismatch which is considered reflective of hibernating myocardium. At angiography the patient was found to have an occluded bypass graft and a second bypass was performed. Six months later the patient was asymptomatic and the LV ejection fraction had increased to 47%. Case courtesy of CTI, Inc. |
|
In the Immediate Post Infarct Period:
In the immediate post infarction period (out to 1-week post event) the functional outcome of infarcted segments with a flow-metabolism mismatch pattern is more variable and does not necessarily imply recovery of contractile function following revascularization (ie: in the post infarct period PET may overestimate the potential for recovery) [8].
Limitations of PET metabolic imaging:
Limitations of FDG imaging include limited data in diabetic patients and recent reports that suggest that increased FDG accumulation can be seen in acute evolving myocardial infarctions [1, 11]. In the immediate post infarction period (out to 1-week post event) the functional outcome of infarcted segments with a flow-metabolism mismatch pattern is more variable and does not necessarily imply recovery of contractile function following revascularization (for example, in the post infarct period PET may overestimate the potential for recovery) [8]. Also, in the evaluation of FDG-PET for myocardial viability there is wide variability in the literature regarding patient inclusion criteria and examination protocol [14].
Other Approaches to Metabolic Myocardial Imaging
In addition to standard perfusion-metabolic imaging using 18FDG, other approaches have also been utilized to assess for viability, including determination of oxidative metabolism with 11C palmitate, uptake and retention of 82Rb, 18F fluoroisonidazole imaging, and the water-perfusable tissue index.
PET Metabolic Myocardial Fatty Acid Imaging
Fatty acids are the major source of energy for the myocardium- long chain fatty acids account for 90% of the myocardial energy requirements in the fasting state.. Fatty acid oxidation generates about 6 times the ATP of glycogen oxidation [59]. When glucose or insulin levels are high, such as after a meal, glucose oxidation increases and fatty acid use is suppressed [2]. During ischemia, glucose again plays a major role in oxidative metabolism, whereas oxidation of long chain fatty acids is greatly reduced [2]. Free fatty acids are transported in the blood bound to albumin [59]. Catecholamines increase the blood concentration of fatty acids [16]. Myocardial uptake and retention of fatty acids has been shown to be affected by ischemia [59]. During ischemia, fatty acid metabolism is affected in a matter of seconds [58].
C-11 palmitate
Carbon-11 is a cyclotron produced positron emitter with a half-life of 20.4 minutes and a positron range of 0.4 mm [44]. 11C can be labeled to long-chain fatty acids to study myocardial metabolism [44].
Long-chain free fatty acids easily pass through the sarcolemmic membrane to be activated as acylcoenzyme A (CoA). Acyl CoA is carried into mitochondria through an acyl carnitine carrier system to enter the beta-oxidation pathway. After beta-oxydation , acetyl CoA is formed and enters into the tricarbonic acid cycle for further oxidation to become water and carbon dioxide. Palmitic acid comprises approximately 25 to 30% of the circulating fatty acid in the blood and serves as one of the primary sources for energy production by the heart. The distribution of C-11 labeled palmitic acid reflects efficiency of myocardial energy production.
After initial uptake of the tracer, there is a rapid clearance component which corresponds to levels of beta-oxidation. This is followed by a slower clearance component which reflects incorporation of the tracer into triacylglycerols and phospholipids.
In normal myocardium, there is homogeneous distribution of the tracer. The half-time of washout after extraction is about 12-18 minutes in the normal heart- reflecting the pathway of tracer uptake, beta-oxidation, and efflux of the end product (CO2) [58]. During periods of ischemia, the uptake of the agent and the rate of the rapid clearance phase and its magnitude markedly diminish, consistent with diminished oxidative metabolism. Delayed clearance is also observed from ischemic and infarcted regions as a result of decreased fatty acid oxidation. C-11 palmitate exams reveal segmental reductions of blood flow in acutely ischemic myocardium are associated with delayed clearance of 11C-palmitate and an increase in 18-FDG uptake.
The rapid blood clearance of this agent and loss of radioactivity from myocardial tissue due to straight-chain fatty-acid catabolism by beta oxidation complicate data analysis. A drawback of this agent is that it is affected by plasma substrate concentrations--in the fasting state, when glucose levels are low, fatty-acid metabolism is accelerated and C-11 palmitate washout is rapid. Washout decreases markedly with glucose loading. The critical organ is the liver.
1-[11C] acetate
Another agent, 1-[11C] acetate can also be used to assess overall oxidative metabolism. The tracer is well extracted by the myocardium and is then converted to acetyl-CoA. Acetyl-CoA is the entry point for all metabolic pathways into the tricarboxylic acid cycle within the mitochondria. Oxidation of acetyl-CoA leads to the production of C-11 labeled CO2 reflecting the activity of the tricarboxylic acid cycle that, under normal conditions, reflects overall oxidative metabolism (i.e: the efflux of labeled CO2 after the administration of C-11 acetate reflects myocardial oxygen consumption [58]).
Even in the face of changing patterns of myocardial substrate use, the clearance of 1-[11C] acetate accurately delineated overall oxidative metabolism (ie: unlike C-11 palmitate, this agent is not affected by plasma substrate concentrations). The maintenance of oxidative metabolism in jeopardized myocardium was identified as a prerequisite to recovery of function following revascularization. Myocardial uptake and clearance are homogeneous in normal subjects. In infarcted myocardium, there is decreased uptake and clearance of the tracer due to the reduced oxygen consumption.
The predictive accuracy of this tracer in viability assessment may be superior to FDG. In one study, 15% of nonviable myocardial regions demonstrated increased uptake of FDG relative to flow, but diminished levels of oxidative metabolism, while 20% of viable segments (demonstrating normal oxidative metabolism) had reduced FDG accumulation [11].
Rubidium-82
For Rb-82 imaging in the evaluation of myocardial viability there is unfortunately no association between the severity of a fixed defect on 82Rb imaging and the likelihood of myocardial viability in that area. In defects containing less than 50% relative activity, 30% were shown to be viable by FDG imaging [13].
18F-Fluoromisonidazole
18F-Fluoromisonidazole has been shown to have increased retention in hypoxic, non-infarcted myocardium. It can therefore be useful for the selective delineation of hypoxic, but viable tissue.
H2150 Water
(H215O) can be used to determine the water-perfusable tissue index. Contractile function is thought to improve after revascularization only if the perfusible tissue fraction is at least 70%.
REFERENCES:
(1) Radiographics 1999; Jadvar H, et al. SPECT and PET in the
evaluation of coronary artery disease. 19: 915-926
(2) J Nucl Med 2000; Tamaki N, et al. The role of fatty acids in cardiac imaging. 41: 1525-1534
(3) J Nucl Med 1994; Schwaiger M. Myocardial perfusion imaging with PET. 35: 693-98
(4) J Nucl Med 1995; de Jong RM, et al. Posterolateral defect of the normal human heart investigated with nitrogen-13-ammonia and dynamic PET. 36: 581-585
(5) J Nucl Med 1998; Wallhaus TR, et al. Human biodistribution and dosimetry of the PET perfusion agent Copper-62-PTSM. 39: 1958-1964
(6) J Nucl Med 1995; Delbeke D, et al. Rest myocardial perfusion/metabolism imaging using simultaneous dual-isotope acquisition SPECT with technetium-99m-MIBI/fluorine-18-FDG. 36: 2110-19
(7) J Nucl Med 1994; Schelbert HR. Metabolic imaging to assess myocardial viability. 35(4 Suppl):8S-14S. Review
(8) J Nucl Med 1995; Grandin C, et al. Delineation of myocardial viability with PET. 36(9):1543-52
(9) J Am Coll Cardiol 1992; Eitzman D, et al. Clinical outcome of patients with advanced coronary artery disease after viability studies with positron emission tomography. 20: 559-65
(10) J Nucl Med 1994; Maddahi J. Cardiovascular nuclear medicine: state-of-the-art. 35(4):672-3 (No abstract available)
(11) J Nucl Med 1994; Bergmann SR. Use and limitations of metabolic tracers labeled with positron-emitting radionuclides in the identification of viable myocardium. 35(4 Suppl):15S-22S. Review
(13) Radiology 1995; Go RT, et al. Hibernating myocardium versus scar: severity of irreversible decreased myocardial perfusion in prediction of tissue viability. 194:151-55
(14) Semin Nucl Med 2000; Bax JJ, et al. 18-Fluorodeoxyglucose imaging with positron emission tomography and single photon emission computed tomography: Cardiac applications. 30: 281-298
(15) J Nucl Cardiol 2001; Dilsizian V, et al. Fluorine-18-deoxyglucose SPECT and coincidence imaging for myocardial viability: Clinical and technical issues. 8: 75-88 (No abstract available)
(16) J Nucl Med 1993; Schelbert H, et al. Positron statement: Clinical use of cardiac positron emission tomography. Position paper of the cardiovascular council of the society of nuclear medicine. 34: 1385-88 (No abstract available)
(17) J Nucl Med 1993; Garcia EV, et al. What should we expect from cardiac PET? 34: 978-980 (No abstract available)
(18) J Nucl Med 2001; Zhang X, et al. Clinical outcome of patients with previous myocardial infarction and left ventricular dysfunction assessed with myocardial 99mTc-MIBI SPECT and 18F-FDG PET. 42: 1166-1173
(19) J Nucl Med 1998; Bax JJ, et al. Comparison of fluorine-18-FDG with rest-redistribution thallium-201 SPECT to delineate viable myocardium and predict functional recovery after revascularization. 39: 1481-1486 (No abstract available)
(20) J Nucl Med 2001; Vitale GD, et al. Myocardial glucose utilization and optimization of 18F-FDG imaging in patients with non-insulin-dependent diabetes mellitus, coronary artery disease, and left ventricular dysfunction. 42: 1730-1736
(21) J Am Coll Cardiol 1997 Bax JJ, et al. Accuracy of currently available techniques for prediction of functional recovery after revascularization in patients with left ventricular dysfunction due to chronic coronary artery disease: Pooled comparison data. 30: 1451-60
(22) J Nucl Cardiol 2002; Bax JJ, et al. Safety and feasibility of cardiac FDG SPECT following oral administration of Acipimox, a nicotinic acid derivative: comparison of image quality with hyperinsulinemic euglycemic clamping in nondiabetic patients. 9: 587-593
(23) J Nucl Med 2003; Schinkel AFL, et al. Dobutamine-induced contractile reserve in stunned, hibernating, and scarred myocardium in patients with ischemic cardiomyopathy. 44: 127-133
(24) J Nucl Cardiol 2003; Kaul S. What lies beyond a chronically occluded coronary artery, and what should we do about it. 10: 92-94 (No abstract available)
(25) J Nucl Med 2003; Schinkel AFL, et al. Effect of diabetes mellitus on myocardial 18F-FDG SPECT using acipimox for the assessment of myocardial viability. 44: 877-883
(26) J Nucl Med 2003; Knesaurek K, et al. Comparison of 2-dimensional and 3-dimensional 82Rb myocardial perfusion PET imaging. 44: 1350-1356
(27) J Nucl Cardiol 2003; Bacharach SL, et al. PET myocardial glucose metabolism and perfusion imaging: part I- guidelines for patient preparation and data acquisition. 10: 545-556 (No abstract available)
(28) J Nucl Cardiol 2003; Schelbert HR, et al. PET myocardial perfusion and glucose metabolism imaging: part 2- guidelines for interpretation and reporting. 10: 557-571 (No abstract available)
(29) J Nucl Cardiol 2004; Beller GA, Bergmann SR. Myocardial perfusion imaging agents: SPECT and PET. 11: 71-86 (No abstract available)
(30) J Nucl Med 2004; van der Weerdt AP, et al. Postinjection transmission scanning in myocardial 18F-FDG studies using both filtered backprojection and iterative reconstruction. 45: 169-175
(31) J Nucl Med 2004; Koepfli P, et al. CT attenuation correction for myocardial perfusion quantification using a PET/CT hybrid scanner. 45: 537-542
(32) J Nucl Med 2004; Loghin C, et al. Common artifacts in PET myocardial perfusion images due to attenuation-emission mis-registration: clinical significance, causes, and solutions. 45: 1029-1039
(33) J Nucl Cardiol 2004; Rimoldi OE, et al. Positron emission tomography for quantification of myocardial perfusion. 11: 482-489
(34) J Nucl Cardiol 2004; Maddahi J. Factors influencing predictive value of FDG imaging for evaluating myocardial viability. 11: 526-526
(35) J Nucl Cardiol 2004; Santana CA, et al. Incremental prognostic value of left ventricular function by myocardial ECG-gated FDG PET imaging in patients with ischemic cardiomyopathy. 11: 542-550
(36) J Nucl Med 2004; Marshall RC, et al. Kinetic analysis of 18F-fluorodihydrorotenone as a deposited myocardial blood flow tracer: comparison to 201Tl. 45: 1950-1959
(37) J Nucl Cardiol 2004; Di Carli MF. Advances in positron emission tomography. 11: 719-732
(38) J Nucl Med 2005; DiFilippo FP, Brunken RC. Do implanted pacemaker leads and ICD leads cause metal-related artifact in cardiac PET/CT? 46: 436-443
(39) J Nucl Med 2005; Namdar M, et al. Integrated PET/CT for the assessment of coronary artery disease: a feasibility study. 46: 930-935
(40) J Nucl Cardiol 2005; Hickey KT, et al. An improved model for the measurement of myocardial perfusion in human beings using N-13 ammonia. 12: 311-317
(41) J Nucl Cardiol 2005; Herrero P, Gropler RJ. Imaging of myocardial metabolism. 12: 345-358
(42) J Nucl Med 2005; Chow BJW, et al. Prognostic significance of dipyridamole-induced ST depression in patients with normal 82Rb PET myocardial perfusion imaging. 46: 1095-1101
(43) J Nucl Med 2005; Stankewicz MA, et al. Myocardial viability assessment by PET: 82Rb defect washout does not predict the results of metabolic-perfusion mismatch. 46: 1602-1609
(44) Radiol Clin N Am 2005; Takalkar A, et al. PET in cardiology. 43: 107-119
(45) J Nucl Cardiol 2006; Thompson RC, Cullom SJ. Issues regarding radiation dosage of cardiac nuclear and radiography procedures. 13: 19-23
(46) J Nucl Cardiol 2006; Bateman TM, et al. Diagnostic accuracy of rest/stress ECG-gated Rb-82 myocardial perfusion PET: comparison with ECG-gated Tc-99m sestamibi SPECT. 13: 24-33
(47) J Nucl Cardiol 2006; Bengel FM. Positron emission tomography and magnetic resonance imaging in heart failure. 13: 145-149
(48) J Nucl Cardiol 2006; Le Meunier L, et al. PET/CT imaging: effect of respiratory motion on apparent myocardial uptake. 13: 821-830
(49) J Nucl Med 2007; Martinez-M?ller A, et al. Artifacts from misaligned CT in cardiac perfusion PET/CT studies: frequency, effects, and potential solutions. 48: 188-193
(50) J Nucl Med 2007; Dorbala S, et al. Value of vasodilator left ventricular ejection fraction reserve in evaluating the magnitude of myocardium at risk and the extent of angiographic coronary artery disease: a 82Rb PET/CT study. 48: 449-358
(51) J Nucl Med 2007; Bacharach SL. PET/CT attenuation correction: breathing lessons. 48: 677-679
(52) J Nucl Med 2007; Di Carli MF, et al. Clinical myocardial perfusion PET/CT. 48: 783-793
(53) J Nucl Med 2007; Alessio AM, et al. Cine CT for attenuation correction in cardiac PET/CT. 48: 794-801
(54) J Nucl Med 2007; Cook RAH, et al. Respiration-averaged CT for attenuation correction in canine cardiac PET/CT. 48: 811-818
(55) J Nucl Med 2007; Merhige ME, et al. Impact of myocardial perfusion imaging with PET and 82Rb on downstream invasive procedure utilization, costs, and outcomes in coronary disease management. 48: 1069-1076
(56) J Nucl Med 2007; Gould KL, et al. Frequent diagnostic errors in cardiac PET/CT due to misregistration of CT attenuation and emission PET images: a definitive analysis of causes, consequences, and corrections. 48: 1112-1121
(57) J Nucl Med 2007; Schinkel AFL, et al. Assessment of myocardial viability in patients with heart failure. 48: 1135-1146
(58) J Nucl Cardiol 2007; Bergmann SR. Imaging of myocardial fatty acid metabolism with PET. 14: S118-24
(59) J Nucl Cardiol 2007; Eckelman WC, Babich JW. Synthesis and validation of fatty acid analogs radiolabeled by nonisotopic substitution. 14: S100-109
(60) J Nucl Cardiol 2007; Di Carli MF, et al. Relationship between CT coronary angiography and stress perfusion imaging in patients with suspected ischemic heart disease assessed by integrated PET-CT imaging. 14: 799-809