CT-guided radiofrequency ablation of lung tumors produced outstanding results in patients with inoperable stage 1A non-small cell lung cancer (NSLSC), Italian researchers reported at the 2004 RSNA meeting in Chicago.
However, a group from Los Angeles found disease progression at the RFA site in 19% a year after treatment in mixed-stage NSLSC patients. More research is needed to determine whether RFA will fulfill its early promise as a reliable, sole treatment for patients with unresectable tumors, the California team reported.
A study of 126 inoperable lung cancer patients, also presented at RSNA, found few problems associated with RFA, a still-experimental procedure that is becoming the treatment of choice for such patients, whose numbers are rising due to the growth of lung-screening efforts in recent years.
In the first study, Dr. Riccardo Lencioni from the University of Pisa in Italy discussed his preliminary results from an ongoing multicenter study that treated 14 patients (12 men and two women, ages 50-74, mean age 66 years ± 7) with inoperable biopsy-proven stage 1A non-small cell lung cancer and who were also considered unfit for radiation therapy. The tumor had to be located at least 1 cm from major airways or blood vessels, and patients could have no substantial impairment of coagulation.
The procedure was conducted under conscious sedation or general anesthesia, using an RFA device consisting of a 150-watt generator, temperature monitor, and expandable multitined electrodes (Starburst XL, RITA Medical Systems, Mountain View, CA).
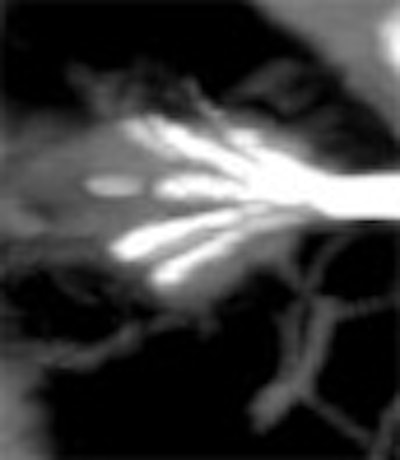
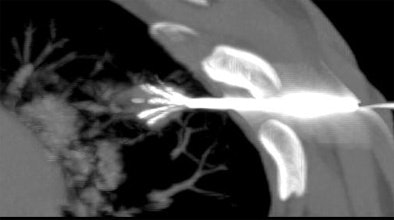 |
| Above and below: Three-dimensional reconstructions of multidetector spiral CT datasets show correct placement of the radiofrequency needle into the tumor. Images courtesy of Dr. Riccardo Lencioni. |
 |
"The concept was to destroy 1 cm of pulmonary tissue all around the tumor. This is why we deployed a maximum 5-cm (ablation target for) a lesion size of 3 cm" (1.0-3.0 cm, mean 2.2 cm ± 7), Lencioni said. "The RFA protocol, in terms of the time and temperature, was prolonged as much as 27 minutes to ensure full ablation of the 5-cm sphere."
The procedure was a technically successful in all 14 patients, and "there were no treatment-related deaths or life-threatening events," Lencioni reported, though there were three pneumothoraces, one of which required substantial drainage. Moreover, he said, "there were no signs of decline in pulmonary function, and the tests that we performed did not show any worsening in this regard."
CT follow-up occurred one and three months after the procedure and at three-month intervals thereafter. Eleven of 13 lesions followed up for at least six months continued to shrink and had no CT-detectable viable tumor, and complete ablation was confirmed for all eight lesions that were followed for a year or more, he said.
"Here you can see one of the cases with a one-year follow-up. The tumor is replaced by a volume of coagulation, which is in excess with respect to the size of the native lesion, and then at three months, six months, and one year there is progressive shrinkage of the coagulation, with some cavitation detectable on CT," he said.
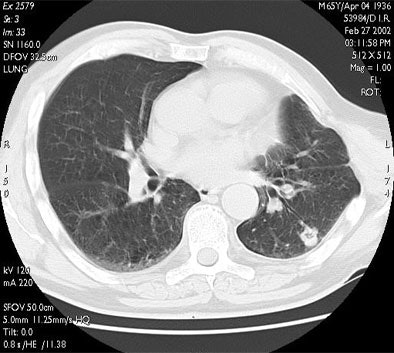 |
| RF ablation of stage IA non-small cell lung cancer (NSCLC). Above: Pretreatment CT scan shows solitary small NSCLC of left lower lobe. Below: At CT obtained one month after RF ablation, a ground-glass density ablation zone replacing the lesion and 1-cm of surrounding pulmonary parenchyma is detected. Images courtesy of Dr. Riccardo Lencioni. |
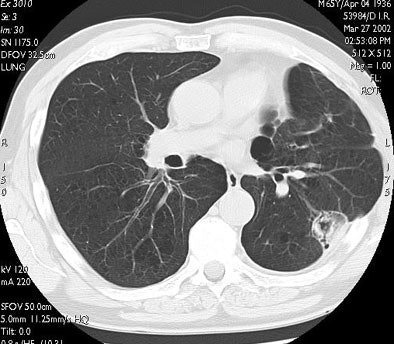 |
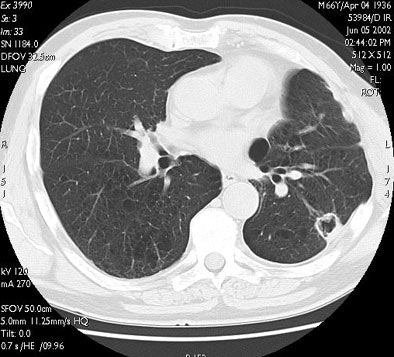 |
| In the same patient, CT studies performed three months (above) and six months after treatment (below) show progressive shrinkage of the area of coagulation necrosis that exhibits central cavitation. No signs of tumor recurrence are detected. Images courtesy of Dr. Riccardo. |
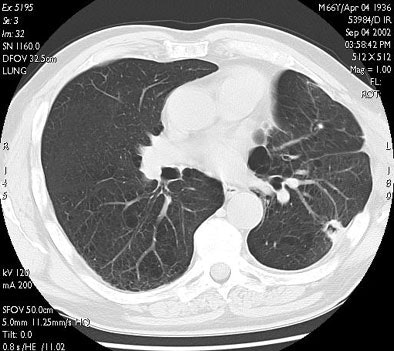 |
A patient followed up for three years showed continued shrinkage of the scarred area, with no sign of relapse. Preliminary results showed overall survival of 71.9% at 12 months, and the substantial comorbidity of the patients accounted for most of the deaths, Lencioni said. No tumor spread was found at follow-up, nor any detectable de-novo cancer following the procedure.
"Actually, only one patient died because of tumor progression, for a one-year and two-year cancer-specific survival of 92%," he said. "Of course, these are very tentative results, but it appears that RFA can provide an effective local tumor control in stage 1A NSLSC patients, with morbidity that seems to be acceptable.... The tentative figures seem to ... compare well with those reported for radiation therapy.... But of course we need further investigation, along with a larger patient cohort, to confirm these promising findings."
Disease recurrence in some NSCLC patients
In a subsequent RSNA presentation, radiologist Dr. Amanda Wallace from the UCLA School of Medicine offered her one-year post-RFA results in a group of NSCLC patients with mixed staging.
"We all know that RFA is an increasingly utilized technique for treating solid malignancies," she said. "Its use in the chest, however, remains experimental."
Wallace, along with Dr. Robert Suh and Dr. Jonathan Goldin sought to evaluate the use of RFA in patients with lesions 5 mm and smaller that were noncontinguous to vital structures and percutaneously accessible, she said. The 21 patients in the study consisted of nine men and 12 women (average age 76), with six with stage 1A NSCLC disease, five stage 1B patients, two stage 2B patients, and one stage 4 patient. Eight had recurrent disease, three of whom had received chemotherapy.
All the patients were found to have unresectable disease following evaluation by a thoracic surgeon or oncologist; contraindications to surgery included unresectability, severe comorbid cardiopulmonary disease, limited recurrent disease following remission, or the patient's refusal to be evaluated for resection. The procedure was performed under conscious sedation, with the RFA probe placed percutaneously into the lesion under CT guidance using a tandem needle technique.
Baseline CT densitometry readings were acquired before CT for the purposes of future comparison, Wallace said. The protocol began with noncontrast CT, followed by postcontrast sequences at 0, 45, 90, and 180 seconds after the 100-cc injection of contrast (Omnipaque 350, GE Healthcare, Waukesha, WI) at 2 cc/sec. The maximal enhancement value at baseline was recorded for use as an index of disease activity. Repeat densitometry studies were scheduled for one, four, six, and 12 months after ablation.
CT images were acquired on a four-slice LightSpeed scanner (GE Healthcare) using 1.25-mm collimation, 120 kVp, 40 mAs, and 0.8-sec. rotation time for the low-dose protocol, and an initial nodule evaluation protocol of 120 kVp and 280 mAs with 0.8-sec rotation. Images were reconstructed using a 0.5-mm interval and a standard reconstruction algorithm.
"Here you see the appearance of a treated lesion of the 12-month study period, as well as lesion enhancement values at the respective time points," Wallace said. "The ablated zone lesion initially appears larger, likely due to local inflammation. The lesion eventually becomes cavitary, and ultimately takes on the appearance of what looks like a scar on CT."
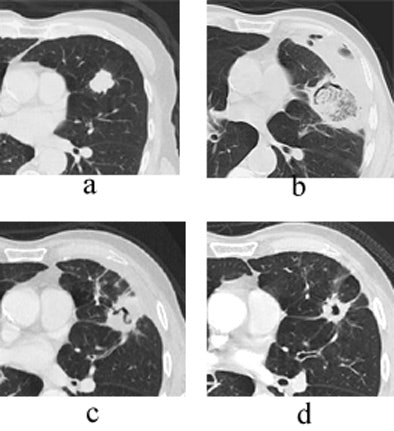 |
| Transverse thin-section CT of a lingular segment nodule prior to RFA and one, three, and six months after RFA. (a) Baseline scan obtained prior to RFA shows a 2.2 x 2.8-cm nodule. (b) Scan one month after RFA shows marked interval increase in nodule size with early formation of a thick-walled cavity and adjacent anterolateral complicated hydropneumothorax. (c) Scan three months after RFA demonstrates interval regression of cavitary lesion and resolving adjacent hydropneumothorax. (d) Scan six months after RFA shows further interval regression of cavitary lesion and resolved hydropneumothorax. Images courtesy of Drs. Robert Suh, Jonathan Goldin, and Amanda Wallace, UCLA School of Medicine, Los Angeles. |
The group's radiologic criteria for progressive disease took the World Health Organization size criteria into account, but due to the difficulty of discerning active tumor from treated necrotic tissue in some cases, the researchers defined a patient with progressive disease having two or more of the following criteria: growth of the RFA site, increasing contrast enhancement, or new sites of disease node enlargement.
"Over the yearlong course of our study, disease progression was observed in 11/21 patients," Wallace said. "Of these, six underwent adjuvant salvage chemotherapy. The sites of disease progression included the RFA site in four cases, regional lymph nodes in two cases, ipsilateral lobe outside the RFA site in one case, malignant pleural effusion in one patient, and extrathoracic disease in four patients."
Four patients died over the course of the study, though no deaths were thought to be related to the RFA, and none had received salvage chemotherapy. Only one of the patients who died showed signs of disease progression.
The most common complication was pneumothorax, with a total of 15 cases, of which four required catheter drainage. Other complications included symptomatic pleural effusion, transient dyspnea, minor contrast reaction, and mild to moderate pain, she said.
"We found that although RFA of lung tumors is relatively safe and technically feasible, there was no clear observed relationship between stage of initial disease and disease post RFA," Wallace said. "In addition, disease progression at one year was no less than that observed for currently available nonsurgical therapies. In other words, as a sole treatment modality for the attempted cure of unresectable NSCLC lung carcinomas, RFA remains questionable."
Wallace had ideas for future studies as well. Because RFA can treat only radiologically visible disease, the use of neoadjuvant chemotherapy to treat microscopic metastases may ultimately improve patient survival, she said. A large prospective randomized trial to compare the efficacy of chemotherapy plus RFA with chemotherapy alone would be particularly useful. And further research is also likely to offer insight into which tumor or patient characteristics can predict success with RFA, she said.
"Methods of post-RFA follow-up still need refinement," Wallace concluded. "With CT alone, it is often difficult to determine whether there is active disease in the tumor bed. The use of PET/CT may ultimately improve our ability to detect disease activity."
By Eric Barnes
AuntMinnie.com staff writer
January 14, 2005
Related Reading
CT-guided RFA effective for local control and palliation of lung cancer, December 1, 2004
CT-guided RF lung cancer ablation successful, October 13, 2004
Radiofrequency ablation shows promise in treating lung cancer, May 14, 2003
RFA shows high success rate in lung tumor ablation, December 2, 2003
Copyright © 2005 AuntMinnie.com