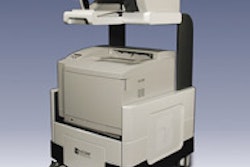
Mirabel Medical plans to show a new version of its electrical impedance scanning device, T-Scan 2000ED, which is designed to serve as an adjunct to clinical breast exam (CBE). Mirabel first introduced its imaging technology in 1999 when it launched its FDA-cleared T-Scan 2000 as an adjunct to mammography.

T-Scan 2000 was intended to help clinicians determine whether women with ambiguous mammography results needed biopsy. Now the Austin, TX-based company has changed its market niche to younger women, ages 30 to 40, based on studies that suggest that CBE's sensitivity is 17%, and that 90% of women who develop breast cancer have no risk factors or history.
T-Scan 2000ED technology is based on the idea that electricity passes through healthy and malignant breast tissue differently. The woman lies on her back and holds a metal cylinder that generates an electric field that the technologist measures by moving a handheld probe across the breast. T-Scan 2000ED creates a real-time electrical image of the breast on a flat-screen monitor. A positive finding does not necessarily mean a woman has breast cancer, but it may mean that she has a higher risk and should be sent to a radiologist for further evaluation.
T-Scan 2000ED is now in clinical studies, according to the company, which is submitting a premarket approval (PMA) supplement for a new market for its original product. Mirabel expects the device to be available fourth quarter of 2005, and to list at about $40,000.
By Kate Madden Yee
AuntMinnie.com staff writer
November 10, 2004
Copyright © 2004 AuntMinnie.com