The recurrence rate in women with ductal carcinoma in situ (DCIS) varies from 5% to 40%, and getting a handle on the progression and timing of DCIS has proved tricky. As a result, the fallback treatment approach has been an aggressive one (usually mastectomy) to head off invasive breast disease.
What's needed is a way to preoperatively characterize DCIS lesions, which would be helpful for surgical planning as well as other nonoperative treatment strategies, according to researchers at the University of California, San Francisco (UCSF). They evaluated whether MRI could reliably capture the size and extent of DCIS, while also correlating imaging features with pathology.
"Mammography detects (DCIS) residue left inside ducts (but) not the disease itself," explained Dr. Laura Esserman and colleagues. "Determining DCIS size on pathology is also a challenge (because) it is difficult to accurately reconstruct the three-dimensional extent using two-dimensional pathology slides.... MRI offers a global picture of extent of disease in the breast and is useful in the local staging of invasive cancer" (Journal of Clinical Oncology, October 1, 2006, Vol. 24:28, pp. 4603-4610).
Esserman and several of her co-authors are from the Carol Franc Buck Breast Care Center at UCSF. Other contributors are from the departments of surgery, pathology, and epidemiology and biostatistics.
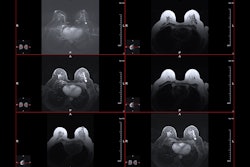
For this study, 45 patients diagnosed with DCIS underwent presurgical, contrast-enhanced MRI on a 1.5-tesla scanner using a 3D, fat-suppressed, fast gradient-echo technique. Maximum anterior-posterior, cranial-caudal, and transverse diameter measurements of lesions were obtained in the enhancing region with the largest lesion diameter recorded as breast MRI tumor size.
Tumor size ranged from 9 mm to 90 mm. The MRI BI-RADS lexicon was used to describe tumor distribution and enhancement patterns. An MRI lesion density score was based on the compactness of pixels, from sparse, stipulated enhancement to solid, mass-like enhancement.
Radiologist Dr. Jessica Leung visually assessed the MR images. Pathologists Dr. Alfred Au and Dr. Yunn-Yi Chen, Ph.D., evaluated all the cases for DCIS features such as nuclear grade, comedo necrosis, size, and density.
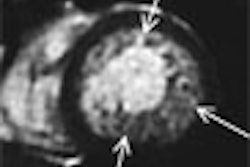
According to the results, MRI size and percentage of involved breast strongly correlated with pathologic size. MR density and enhancement also correlated with pathologic density. Overall, imaging and pathologic features correlated well, "suggesting that MRI measures the same overall set of characteristics that pathologists see in DCIS tumors," such as size, density, and inflammation, the authors stated. However, MRI did overestimate the size of less dense, diffuse DCIS lesions, they noted.
Finally, the compactness of DCIS on a histopathology slide matched the density of pixels enhancing on MRI. On the immunohistochemistry biomarkers, CD68 -- markers that are associated with higher recurrence -- correlated best with MRI features.
"MRI clearly has the capability of providing a three-dimensional map of DCIS. Lesion size on MRI correlated with lesion size at the time of pathologic examination, but heterogeneous lesions are overestimated, perhaps reflecting the presence of a continuum of proliferative changes in noncomedo DCIS lesions," the authors wrote.
Based on these results, Esserman and colleagues stated that they are developing quantitative measures to assess tumor volume and enhancement. The ultimate goal will be to standardize the criteria for DCIS versus high-risk lesions and to distinguish biologic subtypes of DCIS, they explained.
In a second study using the same patient population, Esserman's group further explored the extent to which MRI overestimated DCIS. Out of 45 patients, the team found 10 false-positive cases from patients who had surgical excision based on MRI results.
"These cases were chosen for analysis because there was a clear imaging or palpable correlate of the MRI lesion in question, but the final surgical specimen pathology was not found to be malignant," wrote Dr. Anjali Kumar, the lead author for this study (American Journal of Surgery, October 2006, Vol. 192:4, pp. 520-524).
Among the 45 cases with pathologically confirmed DCIS, overestimation of DCIS size prevailed, the authors stated in their results. MRI overestimated the size of malignancy twofold (≥ 200%) in 13 of 45 cases. The MRI types in these overestimated cases were regional heterogeneous and regional clumped, based on the MRI BI-RADS lexicon.
In addition, when MRI showed enhancement but there was no DCIS malignancy found, 100% of the case showed benign proliferative disease. Of the false-positive MR cases, 80% had benign lesions with an intermediate to high risk for proliferative fibrocystic changes with and without atypia, they stated.
"Where DCIS ends and proliferative changes begin can be difficult to determine," the group concluded. "MRI consistently and accurately reflects pathologic size, except in the presence of proliferative changes that appear to be more common in (estrogen-receptor)-positive noncomedo lesions." Estrogen-receptor-positive DCIS with diffusion enhancement may be amenable to risk reduction with hormone therapy, they added.
The authors also suggested that one strategy for improving the diagnostic accuracy of MRI in DCIS would be to use high-resolution MR along with some type of kinetic analysis designed to assess background parenchyma.
Fine-tuning MRI's ability in DCIS is especially crucial in light of growing genetic evidence that DCIS and invasive breast cancer develop from the same breast cancer progenitor cells.
According to a report at the 2006 International Association for Breast Cancer Research meeting in Montreal, breast cancer progenitor cells, which may be present in precancerous lesions from the start, are genetically programmed to progress to DCIS and possibly to invasive cancer.
The findings are based on studies in a line of transgenic mice engineered to develop mammary intraepithelial neoplasia (MIN), the mouse equivalent of human DCIS. The research was done at the Mutant Mouse Pathology Lab at the University of California, Davis, in Sacramento.
This discovery means that new ways of identifying breast cancer progenitor cells must be found in order to destroy cells before they become malignant, explained lead investigator Dr. Robert Cardiff.
By Shalmali Pal
AuntMinnie.com staff writer
October 18, 2006
Related Reading
Radiation helpful in women with ductal carcinoma in situ, September 27, 2006
Dual-time-point PET spots local, distant breast metastases, September 14, 2006
Copyright © 2006 AuntMinnie.com