Medical imaging is, of course, all about seeing what is generally unseen. Recently, a number of papers have turned the tables, using MRI to study the main organ of sight, the eyes. These studies showed that calling the eyes the "windows of the soul" isn't just a cliché, as they also evaluated the connection between what is seen and what is perceived.
In the first study, investigators from the Jules Stein Eye Institute in Los Angeles shed new light on how the rectus extraocular muscle (EOM) pulleys behave during static ocular counterrolling (OCR), which occurs when the head tilts to the left or right yet the eyes shift in the opposite direction.
In a second study, researchers from three institutions in North Carolina took what they called "an intentional stance" on eye-gaze shifts, using functional MRI (fMRI) to study social perception in children.
Finally, a group from the University of Wisconsin in Madison used fMRI to investigate diminished gaze fixation, one of the core features of autism.
Eye rolling
An otolith is one of the small particles of calcium carbonate in the saccule or utricle of the inner ear. This structure is sensitive to gravity and linear acceleration. Validating otolith function is possible by precisely measuring OCR, so pinpointing OCR measurements is key.
"The existence of orbitally stabilized pulleys implies that the force directions of rectus EOMs change with eye position, and so has important implications for the neural commands required to control eye movements," wrote Dr. Joseph Demer and Dr. Robert Clark from the Jules Stein Eye Institute based at the University of California, Los Angeles (Journal of Neurophysiology, July 20, 2005).
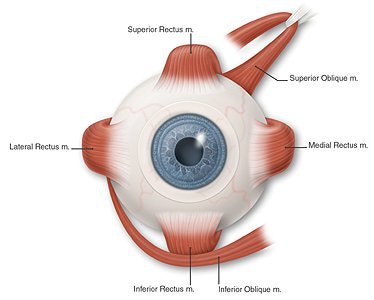 |
| The muscles of the eye. Reprinted from Patient Education product, Ophthalmic Images Collection, volume 3, with permission of the American Academy of Ophthalmology, copyright © 2002. All rights reserved. |
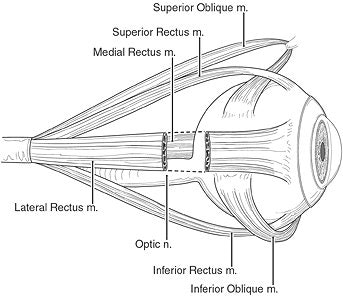 |
Their study evaluated EOM paths and contractility during sustained, onside head positioning. The subjects were 10 adult volunteers who underwent T1-weighted MRI on a 1.5-tesla scanner (Signa, GE Healthcare, Chalfont St. Giles, U.K.) with a dual-phased surface-coil array. Gadodiamide was administered intravenously at a dose of 0.05 mmol/kg.
The participants were scanned both right-side down and left-side down while fixating on a red light that was 2 cm away. The imaging sequences included a triplanar scan, true axial images of both orbits, quasicoronal image sets in the left and right orbits, and quasisagittal images in planes parallel to the long axis of each orbit.
The results showed significant counterrotational repositioning of the rectus-pulley arrays of both orbits in the coronal plane, averaging 4.1 degrees. This was true from both right-side down and left-side down for the inferior, medial, and superior rectus pulleys. In addition, torsional rectus pulley shift during OCR, which changes directions of the rectus EOMs, correlated with known insertions of the oblique EOM orbital layers on rectus pulleys, the authors stated.
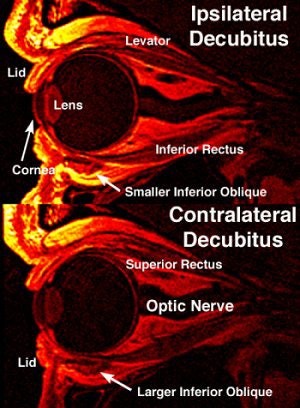 |
| Quasisagittal MRI image ipsilateral decubitus and contralateral decubitus positions. The scans showed contractile thickening of the inferior oblique muscle contralateral to the lower orbit. This was designated image plane 0, the plane where the IO crossed the center of the inferior rectus (IR) muscle. The left of these images is anterior, where the cornea and lens are evident. Image courtesy of Dr. Joseph Demer. |
"The amount of pulley reconfiguration is roughly half of published values of ocular torsion during static OCR, an arrangement that would cause rectus pulling directions to change by less than half the amount of ocular torsion," they wrote.
These results bolster established evidence that pulley torsion and ocular torsion are coordinated, as well as how crucial pulleys are to every aspect of ocular motility.
Shifty-eyed
Previous studies in adults have illustrated that observed eye movements are connected with the superior temporal sulcus (STS) region of the brain. However, little is known about this circuitry in children, according to Dr. Matthew Mosconi and colleagues from Duke University in Durham, NC; University of North Carolina at Chapel Hill; and the Veterans Affairs Medical Center in Durham.
"In this study, we sought to investigate the neural substrates of eye-gaze processing in children and to examine the sensitivity of brain regions involved in processing eye gaze to the intentions underlying shifts in eye gaze," they wrote. "We reasoned that if school-aged children understand the communicative nature of eye movements, then the neural circuitry subserving this process should be sensitive to whether or not eye movements convey a clear goal or intention" (NeuroImage, August 1, 2005, Vol. 27:1, pp. 247-252).
Eight healthy children (ages 7-10; seven females, one male) underwent 50-60 minute sessions on a 1.5-tesla scanner (LX Nvi, GE Healthcare) with a quadrature birdcage radiofrequency head coil. Sixty-eight axial images were acquired using a 3D SPGR sequence. Functional images were obtained using a gradient-recalled inward-spiral pulse sequence.
During imaging, the children watched as a small checkerboard pattern appeared in an animated character's visual field. The experiment had two segments. In the congruent (goal-directed) condition, the animated character shifted her gaze toward the checkerboard. In the incongruent (nongoal-directed) condition, the character shifted her gaze toward empty space, which violated the viewer's expectations.
In terms of a reaction evoked by eye gaze, in both conditions, activity was localized to the right posterior and middle STS regions, including portions of the right superior, middle, and inferior temporal gyri and sulci, according to the results. Regions of frontal lobe activity were localized to the right and left superior, middle, and inferior frontal gyri. The right lateral temporal-parietal cortex, including the posterior STS, responded more strongly to incongruent gaze shifts.
These results were consistent with those found previously in adult studies, the authors stated. They are particularly important as a foundation for continuing studies on eye gaze, neural circuitry, and developmental disorders such as autism, they added.
Eye contact
A core symptom of autism is diminished gaze fixation. Kim Dalton, Ph.D., and colleagues conducted two separate studies with autistic children. Study I looked at emotional discrimination, while study II tested facial recognition. The subjects, both autistic and a nonautistic control group, were shown photos of familiar and unfamiliar faces while undergoing MRI.
Dalton's group is from the Waisman Center and the Waisman Laboratory for Brain Imaging and Behavior, both located at the University of Wisconsin in Madison, as well as the university's psychiatry department.
"The most consistently reported and largest effect is in the fusiform gyrus, an area that is activated strongly during face processing in typically developing individuals but much less activated during these tasks in individuals with autism-spectrum disorders," the group wrote (Nature Neuroscience, April 1, 2005, Vol. 8:4, pp. 519-526).
In addition to hypoactivation in the fusiform gyrus, autistic individuals will show hyperactivation in the amygdala, the area of the brain responsible for processing threatening social and emotional cues.
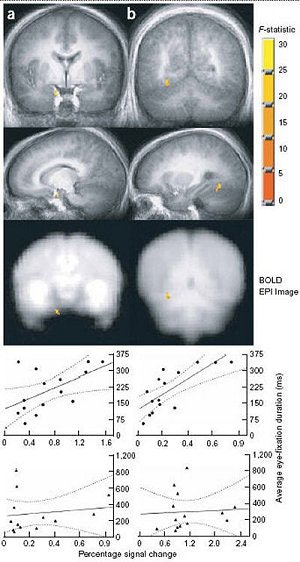 |
| Brain activation clusters associated with average eye fixation time for the autistic and control groups, Study II. Right amygdala (a); right anterior fusiform gyrus (b). The clusters are also superimposed on an averaged EPI BOLD signal illustrating adequate signal coverage for each cluster. Scatter plots showing the relationship between brain activation and average eye fixation are given for each group (autistics, top graphs; controls, bottom graphs) below each cluster. The regression line and 90% confidence bands are superimposed on each scatter plot. Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, Alexander AL, Davidson RJ, "Gaze fixation and the neural circuitry of face processing in autism," Nature Neuroscience, March 6, 2005, p. 5, fig.7. Image courtesy of Nature Neuroscience and Kim Dalton, Ph.D. |
"For both of these brain regions, we predicted that time spent fixating the eye region of the face would predict the magnitude of activation for the individuals with autism," they explained.
The patient population for study I consisted of 14 autistic males (mean age of 15.9 years). Study II was made up of 16 autistic males (mean age of 14.5 years). Eye movements, fixations, and pupil diameter were recorded for both studies using the iView systems with a remote eye-tracking device (SensoMotoric Instruments, Boston).
MR images were acquired on a 3-tesla scanner (Signa, GE Healthcare) equipped with a whole-head, transmit-receive quadrature birdcage head coil. The protocol for study I included 3D, T1-weighted, SPGR volume images; for study II, 3D, T1-weighted, inversion-recovery volume images were obtained. All scans started within 20 minutes of anatomical scans, followed by a seven-minute functional scan during which the subjects performed either the facial emotional discrimination task or the recognition task. The total time in the scanner was no more than 35 minutes.
In both studies, the autistic group spent significantly less time per trial fixating on the eyes than did the control group. The brain activation maps indicated that the control group showed notably greater activation than the autistic group in the bilateral fusiform gyrus, the occipital gyrus, and the middle front gyrus. These areas are associated with face and visual processes. The autistic group also demonstrated greater activation in the left amygdala and the orbitofrontal gyrus.
With regard to the amygdala, the investigators found that activation was strongly associated with the amount of time the autistic group sent fixating on the eyes. They proposed that this diminished gaze fixation might result in the reduction of excessive arousal to social stimuli in autistic individuals.
"These findings indicate that the commonly reported hypoactivation in the fusiform gyrus during face processing in autism may be a function of how individuals with autism scan the face rather than a group difference in which area of the brain is used to process faces," they concluded.
By Shalmali Pal
AuntMinnie.com staff writer
September 2, 2005
Related Reading
Proton beam radiotherapy useful for eye melanoma, August 9, 2005
MRI distinguishes optic neuropathy due to cat scratch disease, July 5, 2005
Ultrasound brings eye imaging into sharp focus, May 25, 2004
Copyright © 2005 AuntMinnie.com