Dutch researchers have found success with a new process that combines multidetector-row CT images with 3D electroanatomic mapping of the heart. A pilot study fused the output of the two modalities to plan catheter ablation procedures in patients with atrial fibrillation.
The fusion was successful, as were the procedures. All 16 patients came out with normal sinus rhythm, the team reported in the October edition of Heart Rhythm.
Catheter ablation aimed at isolating the pulmonary veins can cure the problem of ectopic beats thought to underlie atrial fibrillation. The procedure is performed safely at many institutions, and the results are typically curative, an impressive feat in patients who have not responded to pharmacologic therapy aimed at normalizing heart rhythm.
Before the ablation procedure can be performed, however, detailed information is needed regarding the precise number, location, and anatomy of the pulmonary veins, a task for which CT and MRI have both been tapped successfully.
An additional planning technique known as 3D electroanatomic mapping has also been successful in planning ablation procedures, and can be particularly helpful in complex cases. Electroanatomic mapping depicts electrical activation patterns of the heart in great detail, but anatomic accuracy is not its strong suit. A realistic anatomic representation of the left atrium and pulmonary veins is difficult to obtain. Electroanatomic mapping may miss small pulmonary veins, for example.
The study by Dr. Lauren Tops; Dr. Jeroen Bax, Ph.D.; Dr. Katja Zeppenfeld, Ph.D.; Dr. Monique Jongbloed; and colleagues from Leiden University Medical Center in the Netherlands may be the first to fuse electroanatomic mapping results with multidetector-row CT images.
"Fusions of an anatomic technique with 3D electroanatomic mapping may facilitate ablation procedures," they wrote. "In the current study, the feasibility of fusion of (MDCT) with electroanatomic mapping is demonstrated in patients undergoing radiofrequency ablation for (atrial fibrillation)" (Heart Rhythm, October 2005, Vol. 2:10, pp. 1076-1081).
In the study, 16 patients (15 men, age 54 ± 7 years) with drug-refractory atrial fibrillation underwent imaging on a 64-slice CT scanner (Aquilion 64, Toshiba Medical Systems, Tokyo) within two days preceding radiofrequency catheter ablation.
Images were acquired using 64 x 0.5-mm collimation, rotation time of 400 msec, 100 kVp, and 250 mAs, following injection of nonionic contrast material (Iomeron 400, Bracco, Milan, Italy) at 5 mL/sec. Scan timing was controlled by automated detection of the test bolus in the ascending aorta.
"To assess the number of pulmonary veins, the number of ostia (common or separate ostia) and veins, and the branching pattern of the pulmonary veins, 2D viewing modes and 3D reconstructions were used," the authors wrote. The pretreatment image data were loaded into a 3D electronic mapping system (Carto XP, Biosense Webster, Diamond Bar, CA), equipped with a newly developed image integration module. Previously the accuracy of the registration function was demonstrated in an animal study.
After electroanatomic mapping, a manual registration process is used to fuse the map to the CT images. First, a 3D surface image of the CT data is displayed next to the electroanatomic map, the authors explained.
"A landmark was placed on the electroanatomic map on a certain point (e.g., left atrial appendage) and confirmed by electrocardiac echocardiography and fluoroscopy," Tops and colleagues wrote. "Next, the surface registration algorithm was performed to align the (MDCT) surface image and the electroanatomic map. This algorithm composed the best fit of the two structures based on minimizing distance between the two landmarks and the distance between all mapping points and (MDCT) surface image."
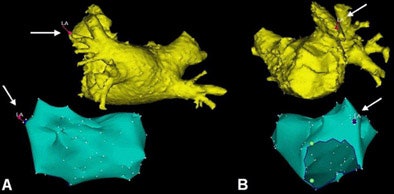 |
| During the ablation procedure, a registration process was performed to fuse the electroanatomic map and MDCT images. After completion of the electroanatomic map of the left atrium, landmarks were placed on the electroanatomic map and the MDCT surface image. In the case above, a landmark was placed on the left atrial appendage, based on fluoroscopy and intracardiac echocardiography. A: posterior view. B: lateral view. The caption has been adapted and the image republished with permission of Heart Rhythm, October 2005, Vol. 2:10, pp. 1076-1081. |
The algorithm measured the accuracy of the registration in terms of mean distance, standard deviation, and range between all mapping points and the MSCT surface image.
Mapping and radiofrequency ablation was undertaken with the goal of obtaining electrical disconnection of all pulmonary veins, using a 4-mm quadripolar mapping/ablation catheter (7 Fr Thermocool, Biosense Webster). The ablation was performed by applying a radiofrequency current outside the ostia of the pulmonary veins using the ablation catheter. The irrigation rate was 20 mL/min, the maximum temperature was 50 degrees C, and the maximum radiofrequency energy was 30 W. Intravenous heparin was administered after the procedure to prevent clotting.
The CT images were accurately fused with the anatomic map in all 16 patients, and image processing of the raw CT data was performed within 10 minutes in all patients, the authors wrote. The fused map and CT data were used to guide the catheter during ablation, performed in a mean procedure time of 89 ± 20 minutes.
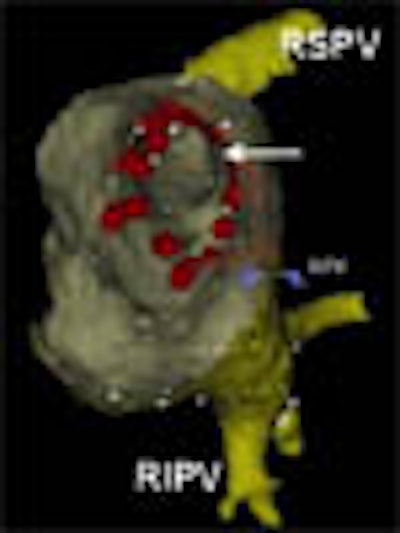
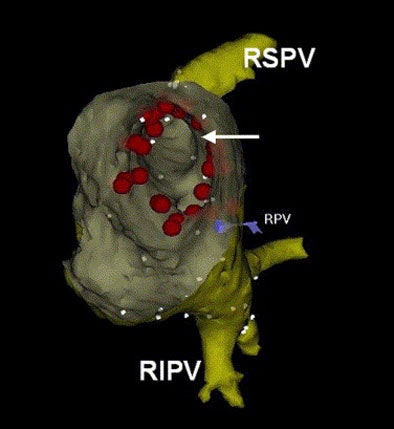 |
| After registration, the fused electroanatomic map and MDCT surface image are used to guide catheter ablation. In the above view from inside the left atrium, the ostium (arrow) of the right superior pulmonary vein is visualized. Radiofrequency current was applied outside the ostia of the pulmonary veins. Red tags indicate sites of radiofrequency current delivery. RIPV: right inferior pulmonary vein; RPV: landmark used for registration. The caption has been adapted and the image republished with permission of Heart Rhythm, October 2005, Vol. 2:10, pp. 1076-1081. |
MDCT identified 67 pulmonary veins (4.2 ± 0.4 per patient), and an additional pulmonary vein on the right side was identified in three patients (19%), and an early branching pattern was observed in the right pulmonary vein. The mean distance between the mapping points and the CT surface ranged from 1.7 ± 1.2 mm to 2.8 ± 1.8, resulting in an average of 2.1 ± 0.2 mm for the patients overall.
"Good contrast timing during computed tomographic scan favored the segmentation process," the team wrote. "The presence of contrast allowed excellent differentiation between the endocardium (low intensity level) and the blood pool (high intensity level). Adequate segmentation of the (CT data) was achieved in all patients."
The study demonstrates that previously acquired MDCT images can be accurately fused with electroanatomic mapping.
"The number and anatomy of pulmonary veins can be highly variable, and the small right middle pulmonary vein has been associated with the initiation of (atrial fibrillation)," the team wrote. "Using the electroanatomic mapping system alone, additional or small pulmonary veins are difficult to identify. Detailed information on number, location, and branching pattern of all pulmonary veins (by MDCT) during the ablation procedure may improve outcome."
However, the study was performed on some patients during breath-hold, while others were breathing normally, the authors cautioned, and heart rate is variable during the procedure. As a result, different volumes of the left atrium during scanning and ablation may limit the image fusion process, though the rapid four-second scan certainly minimizes such problems.
The results prove that accurate fusion is feasible, and may facilitate ablation procedures, the group concluded. "This new technique allows use of real surface anatomy to guide anatomy-based catheter ablation procedures," they wrote. "Fusion of different imaging modalities may facilitate catheter ablation for (atrial fibrillation)."
Dr. Joan Lacomis, associate professor of radiology and a radiologist at the University of Pittsburgh Medical Center, performs a large number of radiofrequency catheter ablations at her facility. In an e-mail to AuntMinnie.com, Lacomis wrote that fusing CARTO electroanatomic mapping results with 3D MDCT images "is reasonable but still in its early stages of development, and warrants further investigation/validation."
"It is another example of how the fusion of different imaging modalities can compliment each other and give additive information over using a single modality alone," she added.
By Eric Barnes
AuntMinnie.com staff writer
November 3, 2005
Related Reading
MDCT edges US for fibrillation RFCA planning, March 2, 2005
Catheter ablation for AF improves outcomes in heart failure, December 2, 2004
RFCA for afibrillation evolves with MDCT, July 23, 2004
TE echocardiography guides treatment of patients with atrial fibrillation, April 18, 2004
Copyright © 2005 AuntMinnie.com



















