A simple threshold-based CT technique may be more accurate than traditional methods of assessing response to targeted therapies in patients with hepatocellular carcinoma (HCC), according to researchers from Boston.
Their new pilot study found that the researchers' proposed method -- measuring non-necrotic tumor volume -- was more accurate than either Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 or total tumor volume for predicting survival in patients with HCC, said Dr. Dushyant Sahani, director of CT and associate professor of radiology at Massachusetts General Hospital.
Considering the nature of cutting-edge therapies such as targeted molecular agents and antiangiogenic drugs, conventional methods of gauging treatment response no longer accurately reflect the true tumor burden, Sahani said in June in a presentation at the International Society for Computed Tomography (ISCT) annual meeting in June in San Francisco.
"As we know, imaging is an accepted surrogate for a variety of chemotherapy results, and the process has evolved," Sahani said.
Evaluating treatment response began with measuring multiple tumors and using the product of two dimensions, per World Health Organization (WHO) criteria, he said. The method was later simplified to use of the longest tumor diameter to assess tumor burden per RECIST criteria. The most recent changes incorporated for the modified RECIST (mRECIST) measurements involve reducing the number of tumor measurements, making it easier for radiologists and others involved in clinical trials to collect measurements of tumor burden.
"This is an easier process, it can be used everywhere, and it's reproducible," Sahani said. "But it doesn't resonate with the treatment available. The challenge is assessing if these patients are getting better response, and if we can predict survival early on within the treatment."
For HCC, available liver-targeted and molecular therapies are generally demonstrating better response and improved survival times as well. But the new treatments don't necessarily reduce total tumor size; in fact, many treatments actually increase tumor size. Instead, by devascularizing large portions of the tumors, the therapies render them unviable.
Newer chemotherapeutic agents for HCC vary in their potential to cause necrosis within the tumors, which has served to highlight the limitations of RECIST criteria. Similar changes can be expected with total tumor volume (TTV), underscoring the need to explore new options, Sahani said.
Measuring enhancement
The limitations of traditional tumor measurement techniques have been recognized by the European Association for Study of the Liver (EASL), which has proposed modifying the 2009 RECIST 1.1 criteria to measure the size of the enhancement rather than total tumor size, Sahani said. Enhancement can be measured as quantitative change in HU on CT or high signal intensity areas on MRI.
But the modified RECIST criteria bring their own complications, Sahani said. Measurements can vary due to subjectivity in lesion assessment and greater influence of having a consistent imaging protocol. In addition, tumor viability measurement based on the diameter of the viable tumor may be challenging in lesions that show partial internal necrosis.
"If the contrast timing is not correct, one can incorrectly characterize the response based on enhancement," he said, noting that study designs using the proposed method are being accepted nevertheless.
Aiming to demonstrate better results in assessing treatment response, Sahani and colleagues proposed gauging treatment response based on viable, or non-necrotic, tumor volume (NNV).
Using CT, "we wanted to investigate if we could actually measure the volume of viable tumor and compare that to different biomarkers, including total tumor volume and RECIST criteria," he said.
Measuring non-necrotic tumor
Sahani and colleagues looked at 63 patients with HCC who underwent conventional treatment in combination with antiangiogenic drugs. A homegrown algorithm was used to differentiate tumor structure and attenuation.
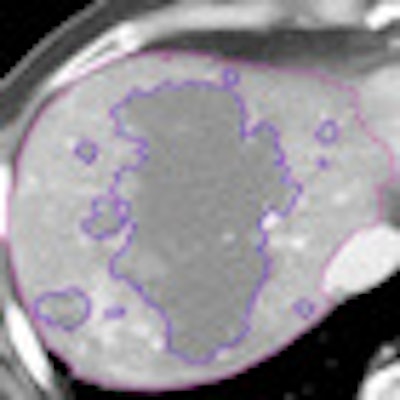
The liver was segmented using a threshold for liver necrosis of about 10 to 30 HU, and a semiautomated technique was used to define the nonenhancing area and tumor structure using a single operator, he explained.
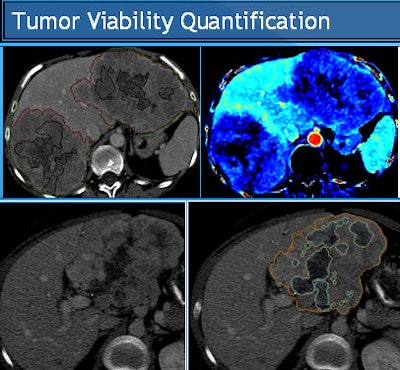 |
| Tumor viability quantification begins with segmentation to estimate total tumor volume and non-necrotic tumor volume. Structure segmentation, extraction, and feature computation are performed for semiautomated/computer-aided detection (CAD) quantification algorithm. Tissues within a defined range of HU values are defined and then margins are outlined, including outer tumor boundaries for TTV; Necrotic portion outlines (10-35 HU). All images courtesy of Dr. Dushyant Sahani. |
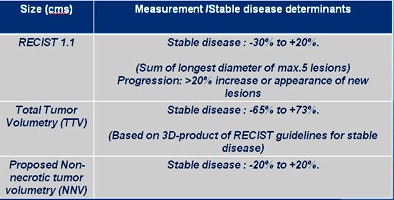 |
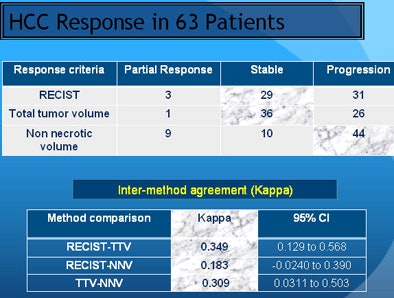
The results varied based on the measurement method. Using RECIST criteria, about half of the patients showed response, while half showed disease progression. But based on total tumor volume, the majority of patients with stable disease end up in the progression category. Using NNV as a measure led to significantly more patients falling into the responder and partial responder categories, he said.
 |
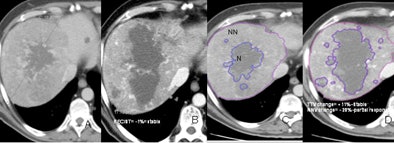 |
| Evaluation of therapy response in a 52-year-old living male with HCC showing a good clinical response to antiangiogenic therapy. Baseline CT scans (A,C) and six months post-treatment CT (B,D) show stable disease according to existing RECIST and TTV criteria but a partial response according to proposed NNV criteria (-28% reduction on NNV). |
Kaplan-Meier analysis showed an overall median cohort survival of 10 months (range, 2-45 months). Per non-necrotic volume criteria, median survival was 36 months for responders and 10 months for nonresponders (p < 0.0001).
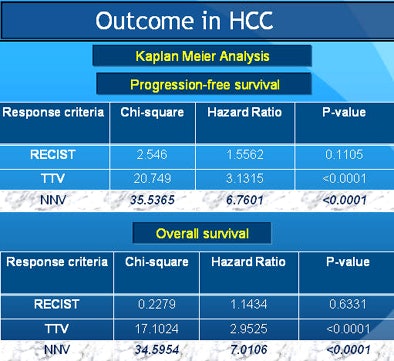 |
"When you look at where the rubber meets the road -- outcomes in patients -- RECIST had the least prediction ability for progression-free survival," Sahani said. "Total tumor volume was a little better, but [non-necrotic] tumor volume had the best response. And both of these numbers are statistically significant."
Univariate logistic regression analysis showed that response assessment by the proposed NNV criteria had a better relation to the overall outcome (odds ratio [OR] = 0.0171; p < 0.0001) compared to TTV (OR = 0.1154; p = 0.0185) and RECIST (OR = 0.3697; p = 0.1688). Stepwise logistic regression analysis revealed NNV criteria as the most optimal method in the study model.
When using newer targeted therapies and liver-directed therapies, oncologists are increasingly demanding image biomarkers," Sahani said. "From our pilot study, we noticed that the conventional methods show less sensitivity in assessing effectiveness and predicting long-term outcomes in HCC."
Both total tumor volume and viable tumor volume can be measured using available software, Sahani said. "In our experience, using homegrown software requires a little work, but it's possible."
"We propose that 20% is sensitive enough for reliably predicting overall response and survival," based on NNV results, he said.
When quantifying tumor viability with this method, he cautioned, it's advisable not to include any major intervening areas of necrosis in the viable tumor diameter.
Sahani said he expects the new method to provide a reliable way of assessing tumor response in HCC clinical trials, but tumor viability measurement needs more studies to validate its use in HCC.
The studies should include larger numbers of patients with HCC, a wider variety of treatments, and tumors other than HCC, he said.