The Medical Imaging and Technology Alliance (MITA) has expressed concern over the U.S. Centers for Medicare and Medicaid Services' (CMS) decision not to include coverage for beta-amyloid PET imaging as part of a new plan to cover Alzheimer's disease drugs.
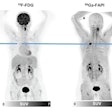
The CMS had announced on June 1 that it will cover U.S. Food and Drug Administration (FDA)-approved Alzheimer's disease drugs for people enrolled in a national patient registry. However, MITA pointed out in a May 30th letter to CMS Administrator Chiquita Brooks-LeSure that the CMS plan fails to provide national coverage to beneficiaries to have the appropriate diagnostic imaging test that confirms or rules out the presence of amyloid beta pathology -- an important element for diagnosis and critical to qualify patients for FDA-approved therapies.
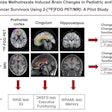
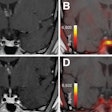
Beta-amyloid PET has become a key tool in the diagnostic workup of dementia and its treatment. Knowledge of amyloid status adds value to the management of patients, minimizes missed diagnoses, reduces the risk of adverse events and potentially inappropriate treatment, and informs decisions that may offer clinical benefit, according to MITA.