An artificial intelligence (AI) model designed for SPECT heart imaging may be able to predict potentially fatal events in patients with cardiovascular artery disease, according to a study published May 1 in npj Digital Medicine.
Researchers at Cedars-Sinai Medical Center in Los Angeles developed a deep-learning model using SPECT myocardial perfusion imaging (MPI) and clinical data from more than 20,000 patients with coronary artery disease. The model performed well in making time-dependent risk predictions and shows promise as a tool for facilitating discussions of possible adverse events with patients, noted first author Dr. Konrad Pieszko, PhD, and colleagues.
"In [common practice], although a patient may be informed that they are at high risk for an adverse event, they are left with less information about what type of event, or within what timeframe can be anticipated, and this can be more unnerving than productive," the group wrote.
Patients suspected of coronary artery disease are often referred for nuclear medicine SPECT scans, which reveal how well blood is flowing to the heart. These MPI exams have proven value for predicting major adverse cardiovascular events on a group level.
But despite the richness and depth of the data acquired, no methods have been established to predict risk of specific events such as death or myocardial infarction based on the MPI exams, the authors noted. To that end, the group sought to leverage deep-learning technology.
The study included 20,401 MPI scans from five international centers participating in a prospective patient registry established in 2018. This registry (Registry of Fast Myocardial Perfusion Imaging with Next Generation SPECT, or the "REFINE registry") was established specifically to aid in the development of new AI tools, the group explained.
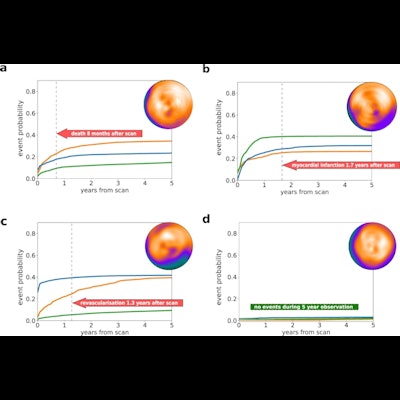
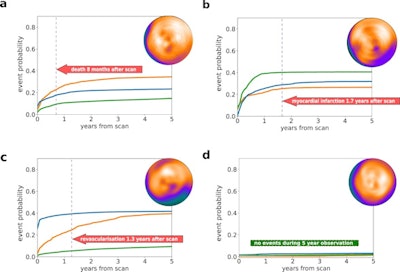 Individual prediction of event probability in four different patients: (a) A 58-year-old male with no history of coronary artery disease (CAD), stress total perfusion deficit (TPD) of 2%, and diabetes; (b) A 76-year-old male with a history of percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG), stress TPD of 3%, and family history of CAD and dyslipidemia; (c) A 63-year-old male with no history of CAD, stress TPD of 20%, and no other risk factors; (d) A 60-year-old male with no history of CAD, stress TPD of 1% and no risk factors. Curves represent predicted cumulative event probability of death (orange lines), acute coronary syndrome (green lines), and revascularization (blue lines) as a function of time. The vertical dashed lines mark the time of the true event. Image and caption courtesy of npj Digital Medicine through CC BY 4.0.
Individual prediction of event probability in four different patients: (a) A 58-year-old male with no history of coronary artery disease (CAD), stress total perfusion deficit (TPD) of 2%, and diabetes; (b) A 76-year-old male with a history of percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG), stress TPD of 3%, and family history of CAD and dyslipidemia; (c) A 63-year-old male with no history of CAD, stress TPD of 20%, and no other risk factors; (d) A 60-year-old male with no history of CAD, stress TPD of 1% and no risk factors. Curves represent predicted cumulative event probability of death (orange lines), acute coronary syndrome (green lines), and revascularization (blue lines) as a function of time. The vertical dashed lines mark the time of the true event. Image and caption courtesy of npj Digital Medicine through CC BY 4.0.As inputs to train the model, the researchers extracted five MPI polar maps (which visualize perfusion, motion, and thickening of heart tissue) combined with 15 clinical features for each case. Follow-up data for each patient detailing times of death or major cardiovascular event classified by type were also included.
The external testing set included images from 13,988 patients who were followed up for a median of three years. Death from any cause occurred in 683 patients (5%) after a median of 1.5 years from the scan. Acute coronary syndrome occurred in 361 (2.5%) patients after a median of 1.3 years and 918 patients (6.6%) underwent revascularization after a median of 0.1 years from baseline imaging.
The model performed best making short-term predictions, the results showed. The model predicted death among patients in the first six months after the scan with an area under the receiver operating curve (AUC) of 0.78, while its predictions in the first six months for acute coronary syndrome achieved an AUC of 0.76.
In addition, the model outperformed conventional perfusion abnormality measures at all time points for death predictions in both the internal and external validation datasets, with improvement increasing gradually over time, the researchers wrote.
"Leveraging a large cardiac imaging registry, we developed a deep-learning approach for individual risk computation that allows time-dependent and event-specific predictions jointly from clinical and cardiac imaging data," the group wrote.
As well, the researchers designed the model to produce visually intuitive graphs for individual risk explanations that they say could be useful for clinicians when discussing MPI results with patients, especially if they have modifiable risk factors.
"By presenting the individualized patient-specific postscan risk assessment over time in an intuitive manner for the clinicians and patients, our approach can potentially help better address patient risk and guide management that is tailored to the patient's individual risk profile," the group concluded.