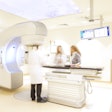
The U.S. Food and Drug Administration (FDA) has cleared superficial radiation therapy provider Sensus Healthcare's Sculptura intraoperative radiation therapy (IORT) system.
Sculptura is used to deliver a single radiation treatment at the point of surgery, which can eliminate weeks of postoperative radiation treatments, the company said. The first system has been installed at the University of Pennsylvania in Philadelphia.