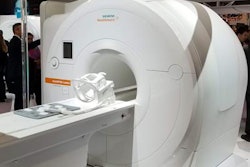
Siemens Healthineers has received U.S. Food and Drug Administration (FDA) clearance for Cios Spin, a mobile C-arm designed to acquire intraoperative 3D images for quality assurance during complicated procedures.
Suitable for use in orthopedic, trauma, and spine surgery, Cios Spin's 3D images can help reduce the revision rate as well as the need for postoperative CT, according to the vendor. It can also support both 2D and 3D imaging for a wide variety of procedures, including vascular imaging, Siemens said. In addition, the vendor has included its NaviLink 3D digital navigation technology.
Cios Spin is available with optional software packages such as Easy 3D, which is designed to provide fast setup and image acquisition, according to Siemens. Another package, Screw Scout, can recognize and automatically label screws on 3D x-ray images.
The system also offers high generator power to accommodate large patients and dense anatomy. In addition, it includes an antimicrobial coating for infection control, the company said.