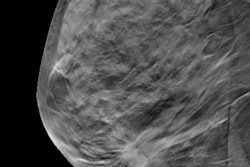
Mammography centers in the U.S. have rapidly adopted 3D mammography -- also known as digital breast tomosynthesis (DBT) -- despite the lack of large-scale clinical trials demonstrating the technology's value, according to an investigation by Kaiser Health News that was published in USA Today.
Mammography vendors, hospitals, doctors, and patient advocates have put millions of dollars into marketing 3D mammograms, the investigation found, leaving "many women feeling pressured to undergo screenings," which, according to the U.S. Preventive Services Task Force (USPSTF), have not been demonstrated to be more effective than traditional mammograms.
"Massive marketing muscle pushes 3D mammograms, despite no evidence they save more lives, investigation shows" is the headline of the article.
Interest in 3D mammography has prompted its swift implementation: Since its clearance by the U.S. Food and Drug Administration (FDA) in 2011, more than 63% of U.S. mammography facilities now offer the technology, according to the report. The exams add about $50 to the cost of a mammogram. When Medicare began to cover 3D mammography, the price increase added up to an additional $230 million on breast cancer screenings within the first three years, the author noted.
Kaiser's investigation outlined the following industry efforts to influence policy and patient care toward 3D mammography:
- Doctor payments: An analysis of data from the Medicare Open Payments database found that 3D mammography manufacturers such as Hologic, GE Healthcare, Siemens Healthineers, and Fujifilm Medical Systems USA have paid doctors and hospitals over $240 million, including more than $9.2 million related to 3D mammograms, in the past six years.
- Patient marketing: Using data from Kantar Media, which tracks the advertising industry, the investigation found that over the past four years, manufacturers spent $14 million to market 3D mammography screening directly to patients.
- Lawmaker lobbying: The report found that private insurers in 16 states are legally required to cover 3D mammography, as are Medicaid programs in 36 states and Washington, DC.
- Grants: According to Hologic's website, the company has bestowed educational grants on the American Society of Breast Surgeons, which has recommended 3D mammography as the preferred screening method.
DBT boosters -- who may also be consultants to the technology's manufacturers -- say they're convinced the technology is better for women, according to the USA Today report.
"When I look at a 2D mammogram now, I don't know how we read them with any degree of confidence," wrote Dr. Liane Philpotts of Yale School of Medicine in a letter quoted by USA Today. "They seem grossly inadequate."
The article noted that "Philpotts' letter did not mention she has worked as a consultant for Hologic, which paid her $13,500 from 2013 to 2018, mostly for research."
The debate over DBT reveals the tension over how much research companies should do before commercializing new products, concluded the USA Today article, quoting Fran Visco, president of the National Breast Cancer Coalition.
"It's incredibly troubling," Visco said. "Everyone has a different stake in all this, and it all seems to be tied to financial gain."